
A Brief History of Elemental Analysis
Elemental analysis by definition covers the whole periodic table and includes both qualitative and quantitative analysis of samples covering a wide range of concentrations. Organic elemental analysis may also be determined for a range of small or large molecule applications. It is possible to analyse a multi-component mixture of compounds using chromatographic techniques. If the mass spectrum of the separated components is determined accurately with high mass resolving power then each compound’s molecular formula may be deduced by software. Inductively coupled plasma mass spectrometry (ICP-MS) or x-ray fluorescence (XRF) instrumentation for the analysis of heavy metals are now ubiquitous in the modern analytical laboratory but what methods were available to chemists several hundred years ago for the determination of elemental composition let alone isotope analysis without a mass spectrometer, was it even possible?
The Early Years
The combustion of a sample to change the colour of a flame must have unwittingly been the oldest method employed for elemental analysis, very little in the way of sample preparation is required. Although not officially recorded Early man must have made these observations since dawn of time when having a fire and inadvertently burning various rocks containing interesting ores. Greek philosophers back in the 3rd century BC proposed that all mater consisted of four elements in nature – earth, water, air and fire. The occupation of Egypt by the Arabs in the 7th century resulted in the first use of the word alchemy in Eurpoe through the combination of ‘al’ from Arabic with ‘Khemia’ the Greek word for Egypt. It was proposed by the Arabian alchemists in the 8th century that all metals were made up of various proportions of mercury and sulfur with gold being the perfect metal. The transmutation of base metals into gold was apparently facilitated by the use of substance known as the pholosphor’s stone. It was not until 1901 Rutherford and Soddy discovered that radioactive thorium was transmutating into radium however Rutherford was apparently not so keen on using the same language as the alchemists.
In 1556 a German metallurgist named Georgius Agricola published the first paper and noted that different ores when placed into a flame changed “the colour of fumes” this information was used for qualitative analysis. In the early 1700s Newton investigated light and developed the prism as a means of separating light into its constituent colour or wavelengths. Another century passed before the prism was used to observe and characterise the spectral lines emitted from the elements present in the sun, Foucault noticed that the sodium lines were present in both the sun and a flame. The collaboration between Bunsen and Kirchhoff who are credited with founding the field of analytical chemistry accelerated the development of a flame excitation instrument by combining the hot Bunsen burner flame with Kirchhoff’s spectroscope. Sample introduction was simple and involved placing small quantities of a solid sample directly into the flame usually on a wire loop. Excited atoms or ions generated in a hot flame emit characteristic photons or coloured light during the radiative de-excitation process. The spectroscope was used to record the wavelengths of the emission lines that were characteristic of the element under investigation, and so optical emission spectroscopy was born. For quantitation purposes it is necessary to measure and record the intensity of the emission for each calibration standard prior to running the unknown sample, this however proved difficult with the technology available at that time. The fundamental research was critical for qualitative analysis but it was a number of years before these prototype instruments matured into a product available on the elemental analysis market for quantitation.
The Flame Photometer
The first invention of an instrument resembling a flame photometer was developed by Champion, Pellet and Grenier in 1873. The instrument consisted of two flames one for the analysis of the unknown sample the other for a calibration standard of known concentration, the operator would view displaced emission spectra from each flame through a spectroscope for a single element sodium. Visual photometry was used to compare the intensity of the emission from the unknown sample with that of the emission from the calibration standard, the concentration of sodium was determined using this method to within 5 %. Their instrument design was later improved by Gouy in 1877, who demonstrated that the intensity of the emission was proportional to the size or temperature of the flame and the amount of sample present. In order to account for this Gouy developed the first pneumatically assisted atomizer for liquid sample introduction, an early ancestor of the modern nebuliser and spray chamber sample introduction techniques, this ensured that the amount of sample entering the flame was controlled therefore improving the accuracy of the analysis. Lundegardh during the 1920s, made further improvements to the sample introduction atomiser for flame photometry instrumentation and also incorporated a photographic plate for recording the results.

Gustav Kirchhoff (left) and Robert Bunsen (right)
Atomic Absorption Spectroscopy
Atomic absorption spectroscopy (AAS) was also developed by Kirchhoff using his spectroscope, he observed that when a bright light was shone through a flame that contained excited atoms of an element, characteristic wavelengths associated with electronic transitions of the element were absorbed leaving dark bands confirming its presence. Kirchoff published “the relation between the powers of emission and the powers of absorption for rays of the same wavelength is constant for all bodies at the same temperature.”
Limitations of the instrumentation required to accurately monitor and record the absorption or emission of light hindered the development of the above spectroscopic techniques for quantitative analysis. The integration of photographic plates by Lundegardh did improve the accuracy and precision but calibration was difficult for absolute quantitation. Spectral interference and a lack of sensitivity of these techniques for certain analytes made them impractical for certain trace analysis applications. The main techniques employed in practice up until the early 20th century and indeed today in some cases for elemental analysis use either gravimetric or titrimetric methods of analysis. What was really needed was the development of a reliable electronic detector for recording the instrument response, once this was achieved a calibration curve could be plotted using external standards.
Following on from Gouy’s initial observations related to the size of the flame and intensity of emission it was later concluded that this relationship was due to an increase in the population of the excited state of the analyte in the flame as a function of temperature. Over the next decade various flames were used including hydrogen in air, acetylene in air and acetylene in nitrous oxide, each of these mixtures produced a hotter flame with an associated increase in detection limit. Flame atomic absorption spectroscopy (FAAS) instruments were developed during the 1950s, for the analysis of aqueous samples, the addition of nitrous oxide acetylene flames improved the sensitivity for certain analytes in challenging environmental samples. The most sensitive AAS instruments available on the market today employ graphite furnaces that can detect an analyte concentration with a detection limit of down to ppb for some assays. Sample preparation often involves microwave digestion of a solid sample in nitric acid, biological samples may also be prepared using a similar method prior to sample introduction at a suitable concentration.
Atomic Emission Spectroscopy
The development of atomic emission spectroscopy did not receive much attention for a number of years due to a lower detection limit being achieved using atomic absorption techniques. A stable plasma source running at more than twice the temperature of the hottest flame was required to realise the full potential of atomic emission spectroscopy. In 1974, the first commercial inductively coupled plasma optical emission spectroscopy (ICP-OES) instruments were developed using argon gas by KONTRON in Germany. The use of inductively coupled argon plasma instrumentation for emission spectroscopy is very popular today and offers a lower detection limit than atomic absorption for elemental analysis especially for the analysis of toxic metals.
Discovery of the Neutron
The only caveat with both of the above aforementioned spectroscopic analysis techniques is their inherent lack of isotopic selectivity. Rutherford and Soddy first proposed the idea of transmutation of the elements back in 1902, that lead to a significant modification to Dalton’s atomic theory however the term or idea of an isotopes was not considered. Some years later Soddy and Fajans simultaneously proposed the existence of isotopes in 1913, through the study of radioactive decay chains and the discovery of three different stable isotopes of lead.
Mass Spectrometry and Isotope Ratio Analysis
Mass spectrometry changed everything for isotope analysis. Thompson and Aston analysed a neon gas sample using a parabola mass spectrograph developed in 1912 at the Cavendish laboratory in the University of Cambridge. Two parabolas or traces were observed when they had both expected only one for the pure sample of neon.
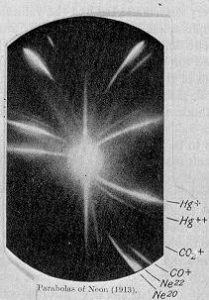
In the bottom right corner of J. J. Thomson’s photographic plate are the separate impact parabola marks for the two isotopes of neon: neon-20 and neon-22.
Thompson had initially thought they had discovered a new element but Aston was not convinced. After learning about Soddy’s discovery and proposed existence of isotopes Aston spent a fellowship distilling liquid air and performing what are now known as isotope enrichment experiments on neon. Neon samples were diffused through porous clay, some enrichment was achieved and measured using a quartz microbalance. Once further developments and improvements by Aston of the mass spectrometry instrumentation came to fruition other isotopes were discovered and the field of isotope ratio mass spectrometry (IRMS) was established. Previous assays using gravimetric analysis for the determination of the (average) atomic mass of the elements on the periodic table were cross validated with results acquired using isotope ratio mass spectrometry with remarkable accuracy. The consequence of the early work carried out by Thompson and Aston was the realisation that the chemical properties of an element are determined by its atomic number rather than its atomic weight. The existence of isotopes was also published independently by Mosley in 1913, who demonstrated the relationship between an element (atomic number) and the wavelength of the corresponding x-ray spectral lines.
Other Methods for Separation of Isotopes
Isotope ratio analysis has really only become a mainstream analytical technique for a variety of sample type due to the use of electromagnetic separation employed by mass spectrometry. The chemical separation of isotopes is difficult because their chemistry is almost identical. Lighter isotopes do tend to react or evaporate more quickly allowing them to be separated however the efficiency is low. Diffusion rates of different isotopes of a gas or liquid across a membrane will be different due to the lighter isotopes traveling more quickly, this method was used by Soddy for his enrichment experiments however the timeframe is such that it is impractical for use as a quick analytical method. Centrifugal separation of isotopes is common for the enrichment of the heavier isotopes containing uranium, the technique was first proposed by Aston and Lindemann back in 1919, again this technique is more suited for large scale preparative applications rather than analysis. Lasers may be used for the analytical separation and detection of a single isotope from a mixture when finely tuned to resonantly excite a hyperfine interaction between an electron and the nucleus, each isotope will have different hyperfine spectra due to the difference in atomic weight of their respective nuclei. Colinear resonance laser spectroscopy (CRIS) when combined with mass spectrometry offers the highest level of sensitivity and selectivity for sub ppq level elemental analysis.
Development of ICP-MS Instrumentation
The realisation that mass spectrometry could be used to determine the mass-to-charge ratio of ions that are generated from an inductively coupled plasma was a huge step change in the development of ultra-trace elemental analysis instrumentation. When compared with ICP-OES analysis the sampling of the ion beam rather than the emitted light offers many advantages, the lack of spectral interference is critical for the analysis of elemental impurities at very low detection limit. The main technological breakthrough came with the development of an atmospheric pressure inlet for a mass spectrometer that could sample the ion beam generated by an argon plasma ion source for subsequent mass analysis.
Sample Preparation and Analysis by ICP-MS
Sample preparation for ICP-MS is the same as the other spectroscopic techniques, aqueous samples are usually prepared in dilute nitric acid. Solid samples may require dissolution in stronger acids such as aqua regia or even hydrofluoric, microwave digestion is also used to speed up the process. Sample introduction involves the nebulisation of the liquid sample into the spray chamber with argon gas. The analysis of a solid sample is also possible with minimal sample preparation using laser ablation, the ablation plume is swept into the argon plasma using a stream of argon gas. Once inside the ICP torch ionization of the sample occurs in the argon plasma to generate the ion beam. The analyte ion will then be selected by the mass analyzer for transmission through to the detector. The most common mass analyzer used in ICP-MS is the quadrupole for both ultra-trace and trace element analysis covering an elemental concentration range of up to 10-orders of magnitude. ICP-MS instrumentation using a single quadrupole mass spectrometer does however suffer from isobaric interference which can mask the analyte ion as a matrix effect. The argon plasma will generate a series of polyatomic interference ions; for example the argon in the plasma will react with oxygen to give argon oxide at m/z 56 which represents a major isobaric interference with the most abundant isotope of iron at the same mass. Chlorine present in a liquid sample can also form an argon chloride polyatomic ion for each of the major chlorine isotopes that constitute a major isobaric interference for the analysis of arsenic. Various methods that have been employed to reduce polyatomic interference from the ion beam the most successful being the use of a collision cell before the quadrupole mass analyzer. The collision cell will fragment the polyatomic interference effectively changing its mass and therefore only let the analyte ion pass through the mass analyzer for detection. Unfortunately, isobaric interference may also present from an atomic ion generated from an isotope of a different element. The only way to remove these interferences is to use a different type of mass spectrometer that incorporates either a high mass resolving power mass analyzer or a tandem quadrupole mass spectrometer with an additional collision cell in between the two quadrupoles. The collision or reaction cell employed for ICP-MS-MS experiments increase the selectivity of the analysis; this is achieved by using a reaction gas that will selectively react with either the analyte or interference ion to increase its mass for subsequent separation and independent detection.
Analysis of Difficult Samples
The most sensitive routine mass spectrometry instrument for ultra trace element analysis in use today that minimise isobaric interference for challenging environmental samples uses a tandem quadrupole mass analyzer ICP-MS/MS. These instruments have a variety of sample introduction inlets available for the analysis of a liquid sample, particles or even nanoparticles in suspension is possible. The addition of an external laser sampling system for mass spectrometry imaging applications of a particular element in a solid sample using laser ablation is also becoming popular. Software now available enables isotope dilution methods to be run with ease and the use of an internal standard is now routine to improve the precision and accuracy of the results. ICP-MS is a highly versatile elemental analysis technique however it is not possible to use it for the analysis of the lighter elements such as carbon. The instrument of choice for isotope ratio analysis would be a mass spectrometer using a magnetic sector with multiple detectors one for each isotope, an inductively coupled plasma ion source or ideally a thermal ionisation mass spectrometer (TIMS) will give the most precise isotope ratio measurements.
Organic Elemental Analysis, the CHNS Elemental Analyzer
Organic compounds are amenable to ICP-MS however they would have to be digested usually by microwave in nitric acid prior to analysis. It is not possible to use an ICP-MS to get any molecular formula information or quantify the ratio of carbon present. If molecular weight information is required one needs to employ a different ionization source for mass spectrometry such as electrospray for LCMS. A simple combustion technique such as CHNS analysis is ideal to determine the ratio of carbon, hydrogen, nitrogen and sulfur in solid or liquid samples. The development of the CHNS elemental analyzer as a commercial instrument for combustion analysis started with Carlo Erba in 1968. Typically, milligram quantities of the sample are introduced into a combustion tube in excess oxygen, flash photolysis ensues followed by gas chromatography to separate and quantify the evolved combustion gas products such as carbon dioxide. CHNS analysis may be used to test product quality, food or soil analysis.
Accurate Mass LC-MS for Elemental Analysis
There are many types of mass spectrometer available today for the analysis of solids, liquids or gases. Liquid chromatography mass spectrometry (LC-MS) involves the hyphenation of a liquid chromatograph with a mass spectrometer. The MS system will usually incorporate an electrospray ionization or atmospheric pressure chemical ionization source. The sample must be soluble in the mobile phase that flows through the liquid chromatograph which is usually a polar solvent. Separation of the analyte from the sample matrix occurs in a chromatographic column due to the analyte’s polarity; the analyte will partition between the polar mobile and immobilized non-polar stationary phase (like attracts like). The stronger the affinity of the analyte to the stationary phase the longer its retention time within the column. Each component or compound separated by the liquid chromatograph will have a unique retention time for a given analytical method run by the liquid chromatograph (mobile phase solvents, flow rate and type of column). Once separated each analyte will flow into the mass spectrometer where ionization will occur, ions generated from the ion source are then separated by the mass analyzer followed by detection to generate a mass spectrum. Electrospray ionization is a soft ionization technique and tends to only yield a protonated molecule in positive ion mode or a deprotonated molecule in negative ion mode. It is possible using tandem mass spectrometry for example to fragment the precursor ion in the collision cell to yield a number of characteristic product ion fragments. The mass spectrometry method development process involves tuning the various ion optics and collision energy to generate a characteristic fragment ion that can be used for quantitation or conformation that an analyte is present in the sample. High sensitivity is one of the best features of MS detection, it is also quick and can be one of the most specific (using accurate mass) and selective analytical techniques available today. Applications include metabolite profiling for metabolomics, proteomics, environmental monitoring and drug development. Once the mass spectrum has been acquired with an accurate mass additional data processing may be used to generate the chemical formula, this in turn will give the elemental composition.
This brief review is by no means exhaustive, there are many other techniques that have played their part in the development of analytical chemistry for elemental analysis. In answer to the original question posed regarding the feasibility of elemental analysis several hundred years ago the answer is yes for certain elements using flame excitation methods, however quantitation was not possible really until the late 1870s. In order to determine the isotope ratio of an element this has only been made possible with the advent of mass spectrometery within the last 100 years. The necessity to push detection limits for the analysis of complex samples has never been in such demand as it is today especially for the detection of radionuclides in difficult environmental samples. The new analytical techniques under development by Artemis Analytical Ltd combine the very best that laser spectroscopy and mass spectrometry have to offer meeting the challenges of tomorrow head on regardless of the complexity of the sample type.
Trackback from your site.



Adina206
| #
I must admit that your post is really interesting. I have spent a lot of my spare time reading your content. Thank you a lot!
Adaline206
| #
I have read your article; it is very informative and helpful for me. I admire the valuable information you offer in your articles. Thanks for posting it.
elavil365y.com
| #
lyrica elavil interactions
A Brief History of Elemental Analysis » Artemis Analytical
http
| #
indomethacin synthesis
A Brief History of Elemental Analysis » Artemis Analytical
amitriptyline7w365.com
| #
gabapentin and amitriptyline
A Brief History of Elemental Analysis » Artemis Analytical
imitrex123.com
| #
imitrex injections generic
A Brief History of Elemental Analysis » Artemis Analytical
mestinon7d24.com
| #
pyridostigmine bromide mestinon is the drug of choice for treatment of
A Brief History of Elemental Analysis » Artemis Analytical
diclofenac24a.com
| #
diclofenac and tramadol
A Brief History of Elemental Analysis » Artemis Analytical
mebeverine3x3.com
| #
mebeverine how to take
A Brief History of Elemental Analysis » Artemis Analytical
pyridostigmine0x7.com
| #
can i order cheap pyridostigmine online
A Brief History of Elemental Analysis » Artemis Analytical
cilostazol77x7.com
| #
cilostazol bleeding time
A Brief History of Elemental Analysis » Artemis Analytical
Aggie206
| #
I see some amazingly important and kept up to length of your strength searching for in your on the site
baclofen7r24.com
| #
can you take hydrocodone with baclofen
A Brief History of Elemental Analysis » Artemis Analytical
maxalt2x2.com
| #
rizatriptan generic maxalt
A Brief History of Elemental Analysis » Artemis Analytical
sumatriptan234.com
| #
can i take sumatriptan after paracetamol
A Brief History of Elemental Analysis » Artemis Analytical
imduraaa.com
| #
getting off imdur
A Brief History of Elemental Analysis » Artemis Analytical
rizatriptan7x24.com
| #
rizatriptan brand india
A Brief History of Elemental Analysis » Artemis Analytical
imuran7www.com
| #
imuran side effects tingling
A Brief History of Elemental Analysis » Artemis Analytical
mobic321.com
| #
can mobic be used for headaches
A Brief History of Elemental Analysis » Artemis Analytical
meloxicam1x1.com
| #
overdose of meloxicam to dog
A Brief History of Elemental Analysis » Artemis Analytical
azathioprine7info.com
| #
azathioprine mmr
A Brief History of Elemental Analysis » Artemis Analytical
piroxicam123.com
| #
dogs piroxicam side effects
A Brief History of Elemental Analysis » Artemis Analytical
lioresalzxz.com
| #
lioresal endikasyonlari
A Brief History of Elemental Analysis » Artemis Analytical
toradol888.com
| #
where can i buy cheap toradol for sale
A Brief History of Elemental Analysis » Artemis Analytical
cyproheptadine24w.com
| #
cyproheptadine 4mg tab
A Brief History of Elemental Analysis » Artemis Analytical
tizanidine01.com
| #
cost of tizanidine without insurance
A Brief History of Elemental Analysis » Artemis Analytical
periactin7s.com
| #
what is periactin tablets used for
A Brief History of Elemental Analysis » Artemis Analytical
artane7y7.com
| #
directions to artane castle shopping centre
A Brief History of Elemental Analysis » Artemis Analytical
ketorolac33.com
| #
ketorolac cheap
A Brief History of Elemental Analysis » Artemis Analytical
zanaflex365.com
| #
zanaflex flexeril together
A Brief History of Elemental Analysis » Artemis Analytical
BrandonLib
| #
Biography of Spanish footballer Pedri https://pedri-bd.com statistics at Barcelona, ??games with teammate Gavi, inclusion in the national team for Euro, meme with Cristiano Ronaldo.
Michaelrew
| #
Строительный и архитектурный портал https://intertools.com.ua все самое интересное о строительстве и архитектуре – новости архитектуры и строительства, обзоры и аналитика.
ScottDup
| #
Latest news league-of-legends-esports.com analytics and forecasts in the world of League of Legends eSports. Stay up to date with all the main events of the professional scene!
Frankdwefe
| #
Invictus Gaming is a legendary invictus-gaming-dota2 esports organization known for its remarkable victories in Dota 2, including the championship at The International 2018.
MichaelSUG
| #
Official website https://t1-league-of-legends.com of the legendary eSports team T1 for League of Legends. Latest news, match results, player statistics and LOL Betting.
Arnoldstunk
| #
Latest news and analytics on League of Legends lol-news matches, tournaments, betting. Stay up to date with the latest eSports events!
Matthewtip
| #
Official website of the T1 t1 lol com League of Legends eSports team. Latest news, matches, statistics, tournaments and predictions on LoL Betting.
Danieledurb
| #
Official website of the award-winning samsung-galaxy-league-of-legends com Samsung Galaxy League of Legends team. Latest news, matches, statistics, player profiles and bets on games.
Ronaldgug
| #
Official website of the eSports organization https://g2-esports-league-of-legends.com
DavidDop
| #
Fnatic is a legendary League fnatic league of legends of Legends eSports team. Our website has all the latest news, matches, player statistics and game predictions.
Thomasexpog
| #
Official Team Liquid website team liquid league of legends latest news, match results, tournaments, player profiles and LOL Betting.
EddieOxype
| #
Gen G is one of the strongest eSports teams gen-g-league-of-legends in League of Legends. Our website has the latest news, matches, statistics and LOL Betting.
DavidDab
| #
Dive into the world of Edward Gaming edward-gaming-league-of-legends com the legendary League of Legends eSports team. Latest news, matches, tournaments, statistics and LOL Betting.
Derekdug
| #
Welcome! Find out the latest news dplus league of legends matches, tournaments and statistics of the Dplus team in the League of Legends!
Ernestnoido
| #
Find out everything about Team WE http://team-we-league-of-legends.com news, matches, player statistics and bets on League of Legends. Support the team!
Albertdaymn
| #
ip camera software software for ip camera recording
Jamesmut
| #
Натуральные молочные продукты https://gastrodachavselug2.ru свежесть и качество с заботой о вашем здоровье! Широкий выбор: молоко, творог, сметана, сыры. Только натуральные ингредиенты, без консервантов и добавок.
Jeweladunk
| #
thc chocolate delivery in prague thc gummies shop in prague
CharlesAddes
| #
weed delivery in prague buy hashish in prague
DavidUlces
| #
kush delivery in prague weed delivery in prague
EdwardFum
| #
thc vape in prague buy thc joint in prague
HarryPal
| #
cannabis in prague hash for sale in prague
EdwardUrite
| #
kush shop in prague thc vape in prague
RobertTar
| #
420 day in prague weed shop in prague
ThomasDus
| #
hemp shop in prague https://smokeinprague.site
BrianPreSt
| #
thc joint in prague 420 day in prague
Elvisdot
| #
cannabis delivery in prague weed store in prague
Richardiroro
| #
Biography of footballer Kylian Mbappe kylian-mbappe-bd.com/ personal life, rumors of an affair with Alicia Aylis and Ines Rau. Career, statistics and salary at Paris Saint-Germain, victory at the World Cup and other achievements of the striker.
WilliamLoomi
| #
Biography of football player Neymar neymar-bd.com/ personal life, relationships and rumors of romances with Katya Safarova, Natalia Barulich, the birth of a son and Bruna’s last girlfriend, the birth of daughters.
AnthonyBut
| #
Biography of Belgian footballer kevin-de-bruyne Kevin De Bruyne (Kevin De Bruyne): personal life, relationship with his wife, conflict with Thibaut Courtois over his girlfriend Caroline.
HarryGof
| #
Biography of football player Luis Alberto Suarez http://luis-suarez-bd.com personal life, daughter, wife, children, height. Leaving the club Atletico Madrid, career in Barcelona and Liverpool, goal statistics.
Michaelsub
| #
Biography of football player Jude Bellingham jude bellingham bd com personal life, relationship with girlfriend Laura. Player statistics in the Real Madrid team, matches for the England national team with Harry Kane, the athlete’s salary, conflict with Mason Greenwood.
ThomasDiels
| #
Try the free demo game Crazy Monkey http://crazy-monkey.com.az (Igrosoft) and read our exclusive review!
Terencewatry
| #
Discover Space XY space-xy.com.az/ Take advantage of bonuses and free play to increase your chances of winning!
JerryGlync
| #
Hot Fruits 100 Slot http://hot-fruits-100.com.az Review by Amatic Industries – Play Hot Fruits 100 demo for free or real money. Bonuses and best casinos for September 2024!
Ralphplumb
| #
JetX is a unique simulation game jetx.com.az/ from SmartSoft Gaming. Players fly a virtual plane and collect their winnings safely.
Alvinorbic
| #
Learn everything about blackjack blackjack com az rules, types of bets, features of the online game and answers to popular questions.
AveryAmicy
| #
Bayer 04 Football Club bayer 04 com az composition, statistics, best Bayer players
MarcusPioro
| #
RB Leipzig http://rb-leipzig.com.az team history, club titles, top scorers and players in team history
AlfredFlaph
| #
Current Heidenheim https://fc-heidenheim.com.az squad with player stats and market value, match schedule, club news and rumours
JeffreyAnymn
| #
Phil Foden phil-foden-az.com is a talented midfielder for Manchester City. Find out about his biography, statistics and latest news.
BradleyFal
| #
Declan Rice (Arsenal) declan rice az com midfielder, 25 years old. Check out his biography, statistics, goals and latest 2024 news.
Michaelnum
| #
Yassin Bunu from Al-Hilal yassine-bounou-az.com biography, statistics, news and everything about his career in football.
Danielraw
| #
Everything about Moussa Dembele moussadembele-az.com/ biography, goals, news, statistics and photos. Follow the career of the Al-Ittihad star with us!
CaseyLit
| #
Thiago Silva thiago silva az com is a legendary defender for Chelsea and the Brazilian national team. On the site you can find a biography, the latest news, statistics, videos and interviews. Learn everything about the career and achievements of the great football player.
Jamesgutle
| #
Learn about Garrett Bale https://gareth-bale-az.com his biography, personal life, achievements and the latest news from the world of football.
RobertKam
| #
Everything about Kaka https://kaka-az.com biography, matches, goals, statistics, photos, videos and the latest news about the football legend on one site!
Wallyeldew
| #
Zico is a legendary Brazilian footballer zico known as “White Pele”. His talent, technique and passion for the game made him an icon of Brazilian football, and his contributions to the sport continue to inspire new generations.
LeonardJah
| #
George Best george-best is a brilliant footballer and a shining symbol of the 1960s, known for his talent and turbulent life. He left an indelible mark on football by combining success on the pitch with the tragedy of personal struggle.
Larrythisk
| #
Rudy Gobert rudy-gobert-az.com is a French center and one of the best defenders in the NBA, nicknamed “The French Tower.” A three-time Defensive Player of the Year, he inspires with his skills and commitment to excellence.
MichaelSweag
| #
Nikola Jokic nikola jokic is a Serbian basketball player, NBA star, and leader of the Denver Nuggets. Known for his unique style of play, court vision, and leadership, he has become a role model for a new generation of centers.
Russellexoma
| #
Luka Doncic lukadoncic-az.com is a Slovenian basketball player, the leader of the Dallas Mavericks team and one of the main stars of the NBA. His unique playing style, records and influence have made him a symbol of European success in world basketball.
Clauderot
| #
Born to a British-Nigerian jamal-musiala-az org father and a German mother of Polish descent, young Jamal explores cultural differences while playing for Germany at the Euros, facing off against Jude Bellingham. Breaking news 2024.
Travisjes
| #
Julius Randle is a versatile NBA forward julius-randle a leader for the Knicks, and an inspiring example of perseverance. His play, leadership, and desire to win make him one of the defining figures in the modern league.
WilliamHog
| #
Cristiano Ronaldo biography cristiano-ronaldo personal life, relationships with Irina Shayk and Georgina Rodriguez, children, career at Real and Juventus, records with Portugal at Euro 2020.
Robertbrask
| #
Biography of footballer Mohamed Salah mohamed salah az org wife Magi Sadiq, children, charity work, book “The Last Pharaoh”. Statistics, salary and awards with Liverpool and Egypt in 2024.
Larrygup
| #
Biography of footballer Luis Alberto Suarez luis-suarez-az.org personal life, wife, children, height. Departure from Atletico Madrid, career at Barcelona and Liverpool, goal statistics. Playing for Gremio, moving to Inter Miami, retirement from international duty and the latest news in 2024.
Horacionig
| #
Spanish footballer and Athletic Bilbao nico williams az org midfielder Nico Williams has had a remarkable career. He shares a close relationship with his brother Inaki. Recent reports have included a potential move to Barcelona in 2024.
JasonCef
| #
Talented Nigerian striker Victor Osimhen victor osimhen proudly represents Italian club Napoli and the Nigerian national team. This story highlights his remarkable sporting career, personal development and notable achievements, including a loan spell at Galatasaray.
KennethPrief
| #
Federico Valverde’s federico-valverde-az org biography: personal life, date of birth, children with Mina Bonino, salary, religion, playing style on the pitch, Real Madrid statistics, salary, number inherited from Kroos, position for 2024 and news.
JamesMus
| #
Biography of Spanish footballer Rodri https://rodri-az.org Manchester City midfielder, salary, worth, religion, Euro match statistics, champion status and games with Joao Cancelo. Latest updates in 2024.
Blakeapabs
| #
Biography of Lamine Yamal lamine-yamal-az.com/ a Spanish winger who plays for FC Barcelona and the national team. Includes career highlights, statistics, Euro 2024 winning salary and personal life.
Frankfat
| #
Magic Johnson’s biography magic-johnson-az.com photos, news, personal life, basketball career, religion, prison, statistics, rivalry with Michael Jordan and his fight against AIDS.
StevenHefly
| #
Biography of LeBron James https://bronny-james-az.org son Bronny James. Covers his personal life, family, health issues, NBA draft, and the latest news in 2024.
MichaelvOp
| #
Frankie de Jong’s biography https://frenkie-de-jong-az.org covers his personal life, height, wife, club stats at Barcelona and Ajax, transfer rumours to Man Utd, playing position and shirt number.
Mathewcop
| #
Luka Doncic biography luka doncic salary, signature shoes, stats, NBA comparisons with Trae Young, games with Jokic and Irving, personal life updates.
DanielHully
| #
Stephen Curry stephen-curry biography: personal life, height, weight, career, team, injuries, three-pointers, LeBron James, statistics, sneakers and 2024 updates.
DavidSwing
| #
Всё для строительства и ремонта https://artpaint.com.ua на одном портале: советы экспертов, обзоры материалов, расчет сметы и готовые решения для вашего дома или бизнеса.
IsaacVoild
| #
Портал о строительстве https://aziatransbud.com.ua статьи, видео, инструкции, каталоги материалов и инструментов. Советы для дома и бизнеса. Легко строить, удобно ремонтировать!
Anthonybeisy
| #
Портал для строительства https://6may.org и ремонта: полезные советы, современные материалы, проекты и идеи. Все, что нужно для воплощения ваших задумок – от фундамента до крыши.
RobertBer
| #
Студия дизайна интерьера https://bconline.com.ua и архитектуры: создаем уникальные проекты для квартир, домов и коммерческих пространств. Эстетика, функциональность и индивидуальный подход – в каждом решении.
RobertFuese
| #
Все об озеленении и благоустройстве https://bathen.rv.ua Ландшафтный дизайн, проекты садов, террас и парков. Идеи для создания зеленых зон, подбор растений и профессиональные услуги для вашего участка.
MichaelVat
| #
стероид бутс drags-masses.com
Jamesrit
| #
CMS 1С-Битрикс Бизнес https://magikfox.ru/catalog/license/upravlenie-saytom/biznes/ купить лицензию в официальном маркетплейсе. Редакция Bitrix Бизнес для интернет магазина. Быстрая отправка лицензионного ключа. Помощь в установке и настройке.
Robertsuert
| #
пневмозаглушка пзу пневмозаглушки
Kevinzef
| #
Ваш путеводитель в мире строительства https://dcsms.uzhgorod.ua идеи, планы, пошаговые инструкции и лучшие материалы. Узнайте, как построить дом мечты или обновить интерьер.
Robertthula
| #
Профессиональный портал для строительства https://blogcamp.com.ua проекты, материалы, расчеты, советы и вдохновение. Все, чтобы ваш ремонт или стройка были успешными.
Davidscoop
| #
Делайте ремонт https://esi.com.ua и стройте легко! Лучшие советы мастеров, подбор инструментов, инструкции и сметы. Мы поможем справиться с любой задачей.
JerryNef
| #
Хотите построить дом https://donbass.org.ua или сделать ремонт? Здесь вы найдете всё: инструкции, идеи, современные технологии и проверенные решения. Портал для тех, кто строит.
Robertdam
| #
Все для строителей и мастеров https://dki.org.ua актуальные технологии, практические советы, строительные материалы и проекты. Простые решения для сложных задач!
Antoniofelty
| #
Создайте дом своей мечты https://intellectronics.com.ua На нашем портале вы найдете идеи, инструкции и новейшие технологии для ремонта и строительства.
JacobRedia
| #
Станьте мастером https://fmsu.org.ua своего дела! Портал для тех, кто хочет строить и ремонтировать качественно и выгодно.
KennethDew
| #
Ландшафтный дизайн https://kinoranok.org.ua и благоустройство для дома, офиса или парка. Профессиональные советы, подбор растений и реализация уникальных зеленых проектов.
WilliamSok
| #
Архитектура и дизайн интерьера https://it-cifra.com.ua под ключ: современные решения, индивидуальный подход и гармония стиля и функциональности. Создаем пространство вашей мечты!
JamesENDOX
| #
Найдите все для ремонта https://keravin.com.ua и строительства! Уникальные идеи, пошаговые инструкции и рекомендации специалистов на одном портале.
Kevinrer
| #
Стройте с комфортом https://mr.org.ua полезные советы, новейшие технологии, пошаговые инструкции и проекты – всё для вашего удобства.
Clintontek
| #
Мы помогаем строить https://juglans.com.ua лучше! Советы, проекты, новейшие материалы и технологии для вашего ремонта или строительства.
Glennzit
| #
Ваш путеводитель в мире строительства https://mtbo.org.ua полезные рекомендации, готовые проекты и современные решения для любых задач.
PeterskerY
| #
Решили строить или делать ремонт https://msc.com.ua Мы подскажем, как выбрать лучшие материалы, спланировать бюджет и воплотить все задумки.
MichaelRoumn
| #
Строительство без лишних вопросов https://okna-k.com.ua наш портал – кладезь информации о современных материалах, технологиях и лучших решениях для дома, дачи или офиса.
CharlesOrgab
| #
Всё для успешного строительства https://newboard-store.com.ua и ремонта на одном портале! Мы собрали актуальную информацию, идеи и инструкции для вашего удобства. Заходите и стройте с нами!
Jasonlek
| #
Все секреты https://mramor.net.ua строительства в одном месте! Советы экспертов, подбор материалов и готовые проекты для вдохновения.
Jimmietwire
| #
Ваш путеводитель в строительстве https://quickstudio.com.ua Ищите материалы, технологии или советы – всё это есть на нашем портале. Стройте с комфортом!
MichaelBal
| #
create a company in montenegro Montenegro temporary residence
ElmerMer
| #
Информация о стройке https://purr.org.ua без лишних сложностей! Наш портал поможет выбрать материалы, узнать о технологиях и сделать ваш проект лучше.
Jamesbooms
| #
1win website when is 1win token listing
HermanPew
| #
1win bet 1win bet
StephenHon
| #
Всё для вашего ремонта https://reklama-region.com и строительства в одном месте! Практичные советы, современные решения и актуальная информация для успешного проекта.
EzekielDob
| #
Всё, что нужно знать о металлах https://metalprotection.com.ua от их свойств до применения в различных отраслях. Обзоры, советы, новости и информация о производителях для вашего удобства.
Kevinhew
| #
Вавада предлагает приложения для ставок на любой вкус! Здесь вы найдете ставки на футбол, теннис, баскетбол, киберспорт и многое другое. Широкий выбор событий, удобный интерфейс и выгодные коэффициенты делают платформу идеальной как для новичков, так и для опытных игроков. Начните свой путь в ставках уже сегодня!
LarryCix
| #
Хотите построить дом https://samozahist.org.ua или сделать ремонт? На нашем портале вы найдёте лучшие решения и вдохновение для вашего проекта.
Richardinjuh
| #
Ищете проверенные строительные советы https://rus3edin.org.ua Наш портал поможет выбрать материалы, спланировать проект и сделать всё на высшем уровне.
Ronaldfoere
| #
Строительный портал https://sinergibumn.com для тех, кто хочет знать больше о строительстве. Актуальные идеи, проверенные технологии и вдохновение для любого проекта.
Danielkam
| #
Всё о дизайне интерьера https://sculptureproject.org.ua в одном месте! Узнайте, как создать уютное, стильное и функциональное пространство, которое будет радовать каждый день.
FrankJeasy
| #
разработка и утверждение программы производственного контроля Москва https://ppk-213.ru
RaymondCar
| #
Экспертный строительный портал https://smallbusiness.dp.ua для вашего проекта! Советы, новинки и инструкции для тех, кто хочет сделать всё идеально.
Stacywem
| #
Хотите стильный интерьер https://sitetime.kiev.ua Наш портал предлагает уникальные идеи, профессиональные рекомендации и примеры лучших дизайн-проектов.
Rogerfar
| #
Строительный портал https://sushico.com.ua для профессионалов и новичков: от выбора материалов до готовых проектов. Легко найти подрядчиков, изучить современные технологии и воплотить идеи в жизнь!
Bretthuh
| #
Найдите всё о строительстве https://srk.kiev.ua и ремонте на нашем портале. Полезные статьи, актуальные технологии и лучшие практики ждут вас.
DavidPen
| #
Преобразите ваш дом https://vineyardartdecor.com вместе с нами! На портале вы найдёте свежие идеи, советы по планировке и материалы для создания идеального интерьера.
Morrisneoro
| #
Планируете стройку https://texha.com.ua или ремонт? У нас вы найдёте проверенных специалистов, инструкции, материалы и проекты на любой вкус. Всё для комфортного строительства!
RonaldHab
| #
Всё о строительстве https://valkbolos.com и ремонте на одном портале! Гид по материалам, обзор инструментов, советы по дизайну и подбор подрядчиков. Создавайте дом своей мечты!
Williamunodo
| #
Ремонт и строительство https://sota-servis.com.ua легко! Здесь вы найдёте инструкции, рекомендации, материалы и специалистов для успешного выполнения ваших задач.
DonaldRet
| #
Планируете ремонт или строительство https://vodocar.com.ua У нас всё, что нужно: от инструкций и советов до подрядчиков и обзоров материалов. Стройте с нами!
Robertofroni
| #
Лучшие советы по строительству https://stroysam.kyiv.ua и ремонту на одном сайте! Найдите вдохновение, изучите обзоры и воплотите свои идеи с профессиональной помощью.
HaywoodtUp
| #
Полный справочник по строительству https://stroy-portal.kyiv.ua и ремонту: советы, инструкции, дизайн-решения и помощь с выбором материалов и подрядчиков.
MichaelJab
| #
Ваш гид в мире строительства https://vitamax.dp.ua и ремонта! Обзоры, практические советы, дизайн-идеи и подбор профессионалов для реализации любых проектов.
SamuelWhisa
| #
Сделайте ремонт https://tfsm.com.ua мечты с нашим сайтом! Советы, инструкции, рейтинг специалистов и новинки строительного рынка для вашего удобства.
ShawnIcoft
| #
Простые решения для ремонта https://teplo.zt.ua и строительства! Идеи дизайна, рекомендации экспертов и проверенные материалы для вашего проекта.
RaymondLeque
| #
Стройте и ремонтируйте https://suli-company.org.ua с лёгкостью! Полезные статьи, инструкции, советы по выбору материалов и подрядчиков ждут вас здесь.
Walterdut
| #
Портал о ремонте и строительстве https://buildingtips.kyiv.ua с полезными статьями, рекомендациями по выбору материалов и подрядчиков.
Nathantes
| #
Полный гид по строительству https://tsentralnyi.volyn.ua и ремонту: от планирования до отделки. Читайте, выбирайте и стройте с уверенностью и комфортом.
Nathantes
| #
Полный гид по строительству https://tsentralnyi.volyn.ua и ремонту: от планирования до отделки. Читайте, выбирайте и стройте с уверенностью и комфортом.
MichaelMonee
| #
ggdrop free spin промокод гг дроп
TimothyDak
| #
Приветствую на https://b2best.at! Мы предлагаем надежные и проверенные покупки в интернете. Ознакомьтесь с нашими статьями о безопасности и легальности. Ваши покупки — наш приоритет!
Cornellref
| #
Tormac.org https://tormac.org – это специализированный торрент-трекер, предназначенный для пользователей Mac-компьютеров. Сайт предоставляет широкий выбор контента, ориентированного на операционные системы macOS и iOS.
SamuelWhisa
| #
Сделайте ремонт https://tfsm.com.ua мечты с нашим сайтом! Советы, инструкции, рейтинг специалистов и новинки строительного рынка для вашего удобства.
MichaelSpino
| #
A detailed history inter-milan-az.com of the Italian football club Inter Milan. From their first Scudetto to their Champions League victory.
kinogo
| #
kinogo
LucasMor
| #
mostbet casino bonus mostbet скачат
Jamesmub
| #
mostbet промокод 2024 mostbet kasino
BryanVar
| #
lucky jet 1win скачать на айфон 1win зарегистрироваться
Chesterguarm
| #
MLB Draft Betting https://bettingblog.website Your guide to the world of MLB draft betting. Expert predictions, top odds and detailed analysis will help you increase your chances of success. Bet and win with us!
AntonioCem
| #
Best Forex Trading Course https://blogforex.tech is your key to successful trading. Learn the secrets of professionals, study strategies and learn how to minimize risks. Master Forex easily and effectively!
RobertWox
| #
Credit Union Mobile Home Loans https://blogcredit.tech are the perfect solution for buying or refinancing a mobile home. Affordable rates, easy application, and reliable support every step of the way. Take the first step toward your home with us!
MichaelTof
| #
Tennis betting https://yourmoneyblog.site best odds, predictions and analytics. Explore detailed match reviews, statistics and strategies to make successful bets. Use our tips and win!
FrancisVaf
| #
Federal Gov Open Enrollment https://body-balance.online is your chance to upgrade or choose an insurance plan. Easy navigation, expert support, and a wide range of programs will help you make the right choice. Apply now!
RobertCef
| #
Crypto Funk https://besttodaynew.com is a fresh look at cryptocurrencies. News, trends, guides and analytics for beginners and professionals. Find out how to get the most out of blockchain technology!
Enriqueusece
| #
TaskMy.ru – профессиональная помощь в решении задач любого уровня
TaskMy.ru – это надежный сервис, который предлагает качественную помощь в выполнении задач любых направлений: от технических расчётов и программирования до написания текстов и аналитики. Мы работаем быстро, эффективно и ориентированы на ваши требования.
Доверяя TaskMy.ru, вы получаете индивидуальный подход, точное соблюдение сроков и доступные цены. Оставьте свою задачу профессионалам – результат превзойдет ожидания!
MarvinNaw
| #
Auto loans from Community Credit Union https://sunnydays100.com are simple, affordable, and great value. Low interest rates and flexible repayment options make it easy to buy a new or used car.
Nelsontaf
| #
Home Equity Loans https://funnydays1.com How They Work, What Are the Terms and Benefits? Get the full details on how to use your home’s value for financial purposes. Find out more today!
Felipenog
| #
Credit score requirements for FHA loans https://lifeofnews1.com minimum threshold and tips for improving. Find out how to increase your chances of getting a loan, as well as what affects approval. Detailed information for those who want to get a mortgage through FHA.
Glenndup
| #
No Credit Check Loans in Abilene TX https://daynewday1.com is fast access to money without unnecessary checks. Convenient terms, simple application and instant approval. Get financial help when you need it!
RobertGer
| #
Размеры картонных https://giftbox-3.ru коробок оптом и печать коробки с логотипом в Братске.
RonaldSmaps
| #
Try your luck at taya365 download, where excitement meets reliability! Hundreds of popular games, unique promotions and instant payouts await you.
Pedrodef
| #
kush for sale in Prague Hashish for sale in Prague
RichardHax
| #
Hash delivery in Prague THC gummies for sale in Prague
BruceVot
| #
Biography of football legend Pele pele-az.com/ personal life, ex-spouses, children and current wife Maria Aoki. A reminder of his legendary career, goals and special style of play, as well as successful performances in the national team.
Larrydix
| #
Paolo Maldini’s biography http://paolo-maldini-az.org photo, defender 2019, personal life, Instagram, Milan, salary, religion and news.
Charleswon
| #
Biography of Spanish footballer Xavi Hernandez http://xavi-hernandez-az.com coaching career, Barcelona statistics, matches with Iniesta and Messi, Guardiola’s influence, goals, youth training, dismissal as Barcelona coach, personal life and 2024 updates.
DustyHok
| #
Discover the life of Sergio Busquets sergio-busquets his parents, his partner Elena Galera Moron, his sons, his club career, his achievements with Spain and the latest news for 2024.
CharlesFUT
| #
Biography of Brazilian footballer Ronaldinho ronaldinho-az.org personal life, son’s contract, current position. Club career, free kick goals, dribbling, jersey number, Ballon d’Or award, prison sentence. Latest news of 2024.
Wendellfloab
| #
Biography of Spanish footballer jordi-alba-az.org Jordi Alba: Left back for Barcelona and the Spanish national team, trained at the academies of Barcelona and Valencia.
Josephinivy
| #
Biography of footballer casemiro-az.org Casemiro: personal life, wife and children. Career, statistics, salary at Real Madrid, Brazil national team, transfer fee, position, transfer to Manchester United and the latest news for 2024.
Timothybob
| #
Welcome to the fan site memphis depay dedicated to the active Dutch footballer Memphis Depay, his career path with clubs and the Dutch national team. Tattoos, personal life and news.
CraigZew
| #
popular manga Dragon Ball free online manga update Naruto free online
JeremyMeelo
| #
James Rodriguez james-rodriguez biography: personal life, latest news, Instagram, goals, transfer to Rayo Vallecano, statistics and captain of the Colombian national team.
Jamesfrott
| #
Nicholas Jackson nicolas-jackson-az com is a Senegalese professional footballer who has taken the football world by storm with his play and achievements.
SpencerImike
| #
Biography Pau Victor https://pau-victor-az.org is a Spanish footballer who started in the lower divisions. Thanks to hard work, he got into a good youth team at FC Barcelona. He played for Girona and has good statistics.
RonaldVerma
| #
Biography of Jamie Bynoe-Gittens jamie-gittens-az.org/ a winger for German club Borussia Dortmund, and an English footballer.
Rogercoott
| #
When Huseyin and Naime hakan-calhanoglu welcomed their son Hakan to Monchengladbach, Germany, during the 1994 World Cup, they had no idea he was destined to become a star. Learn his biography, facts and news.
BennyJarie
| #
Biography of Dominik Szoboszlai dominik szoboszlai az org a Hungarian footballer, midfielder for Liverpool and captain of the Hungarian national team.
RogerJaway
| #
Biography of Ollie Watkins https://ollie-watkins-az.org an English striker for Aston Villa and the England national team. Find out about his career, clubs such as Exeter City and Brentford, height, age, achievements, participation in Euro 2024, personal life and latest news.
WilbertRot
| #
Phil Foden Biography https://phil-foden-az.org A look at his personal life, relationship with Rebecca and the birth of his sons and daughters.
GeorgeSluth
| #
Francisco Conceicao https://francisco-conceicao-az.org was born into a family where football was already a part of the family, with a legacy built on hard work and passion. Juventus, Porto and Portugal national team appearances, brother Rodrigo, FIFA stats and news.
WilliamVok
| #
Biography of Austrian jorginho-az org footballer Arnautovic Marko – games at FC Werder Bremen and Internationale, market value, achievements. Personal life, conflicts, rumors and latest news.
GeorgeInvak
| #
Biography of Austrian footballer marko arnautovic az org Arnautovic Marko – games at FC Werder Bremen and Internationale, market value, achievements. Personal life, conflicts, rumors and latest news.
RobertNeaxy
| #
Biography of Federico Chiesa federico-chiesa-az.org Italian winger for Juventus and the Italian national team. Career, Fiorentina, Euro 2024, transfer to Liverpool, family, wife Lucia Bramanti.
Jasontoini
| #
Aurelien Tchouameni’s aurelien-tchouameni-az.org biography: personal life, date of birth, parents’ jobs, statistics at Real Madrid and Monaco, position, jersey number, 2024 updates.
RonaldHex
| #
Biography of Dayot Upamecano dayot upamecano Bayern star, France national team hero, early career start, Euro play-off participation, personal life and football news for 2025.
Jordannaw
| #
Biography of Portuguese bernardo silva footballer Bernardo Silva: personal life, relationship with his wife, similarities with Bruno Fernandes. Current team, number on the field, reviews of fans who have sold out.
SidneySox
| #
Biography of Brazilian football player Rodrigo rodrygo-az com photos of the striker of the Real Madrid club and the Brazilian national team, sports career.
Michaeltrals
| #
Biography of Spanish pedri footballer Pedri, statistics at Barcelona, ??games with teammate Gavi, joining the national team for the Euros.
Archieplabe
| #
Biography of football player antoine griezmann Antoine Griezmann: personal life, birth of children, national origin. Now his career, playing for Atletico Madrid.
ThomasCig
| #
Biography of Brazilian https://alisson-becker-az.com footballer Alisson: photo of the goalkeeper of the Liverpool club and the national team, sports career, playing for FC Inter and Roma, growth, achievements.
Jamiepef
| #
Нужен ремонт техники чинпочин.рус все услуги для вашего дома в одном месте! Выбирайте мастеров для ремонта, уборки или сантехнических работ. Качественный сервис, прозрачные цены и удобство использования.
RafaelGeala
| #
Tobey Maguire’s tobey-maguire-az com biography: personal life, memories of him, friendship with Leonardo DiCaprio, divorce from ex-wife. Role in Spider-Man films, career now.
Jeremyclear
| #
Biography of Spanish footballer Dani Carvajal http://dani-carvajal-az.com personal life, marriage to Joselu and twin sisters. Performances at the Euro for Real Madrid and the Spanish national team.
AnthonyMab
| #
Biography of footballer https://iker-casillas-az.com Iker Casillas: personal life, separation from ex-wife Sara Carbonero.
MerleDiolo
| #
Biography of actor Keanu Reeves keanu-reeves-az.com/ personal life, the tragic death of his child and his relationship with his common-law wife Jennifer Syme, artist Alexandra Grant.
DavidSox
| #
portable ftp software Newsgroup Clients
Jesustooff
| #
“Ищете качественный кирпич напрямую от производителя? https://Muravey61.ru – ваш надежный поставщик строительных материалов в регионе! Мы предлагаем кирпич высшего качества по доступным ценам прямо с завода. Доставка точно в срок, широкий ассортимент, и гарантированное качество – всё, что нужно для вашего строительства. Закажите у нас и убедитесь сами, что с нами строить легко!”
Josephcew
| #
Now you can’t find a person https://formula1-az.com who hasn’t heard of Formula 1. Today it is one of the most prestigious and popular sports on the planet.
Ronaldcrest
| #
Biography of Zendaya Coleman zendaya maree (Zendaya): modeling career, music and cinema, details of her personal life, ex-boyfriend Jacob Elordi, romance with Tom Holland.
BruceBon
| #
Welcome to the main page karim-benzema-azerbaijan.com of the fansite dedicated to the world football star Karim Benzema. Learn all about his incredible career, incredible achievements and phenomenal skills.
Eugenevat
| #
Welcome to the world of Neymar neymar-azerbaycan com a fan site dedicated to the great footballer. Learn all about his career, achievements and unique playing style.
VernonBiore
| #
Biography of British actress emily blunt az com Emily Blunt: personal life, dating and relationship with her husband John Krasinski, raising children.
Vanilla Gift Card
| #
We genuinely appreciate your guidance and the thoughtfulness you’ve shown. It matters greatly to us.
KelleySoync
| #
Jogo Do Tigrinho slot takes you to the world of exoticism and excitement. Incredible winnings, bonus games and a dizzying jungle atmosphere await you. Bright graphics and exciting gameplay make the game unforgettable. Try your luck right now!
Brandoncab
| #
Welcome to the fan site moussa-dembele-az com of the talented footballer and real star Moussa Dembele! Here you will find everything new and most interesting about his career, achievements and amazing moments on the field.
Nathanhoast
| #
скупка золота спб дорого цена за грамм скупка золота 585
StephenInhit
| #
Оперативная помощь на дороге https://angeldorog.by услуги эвакуатора, грузовой и легковой шиномонтаж, а также грузоперевозки фурами по доступным ценам. Работаем круглосуточно, быстро реагируем и гарантируем надежность. Звоните в любое время – решим вашу проблему!
JefferyRok
| #
CMS software Webcam security camera software
DavidGrite
| #
Discover the ultimate hub zinedine zidane azerbaijan com for all things Zinedine Zidane. Dive into in-depth analysis of Zidane’s illustrious career, from his legendary performances to his coaching triumphs.
ErnestFrazy
| #
Find out all about Kylie Jenner kylie-jenner-azerbaijan com at Kylie Jenner, the ultimate fan site for the latest updates on her fashion line, beauty tips and personal life.
Metamask Download
| #
I’ve been using MetaMask Wallet for years, and recently downloaded the latest MetaMask Extension via https://metalead.org/. It works flawlessly on Chrome and Safari browsers!
RobertKat
| #
Immerse yourself in the world of Gigi Hadid gigi-hadid-azerbaijan.com/ through our dedicated website. Explore the latest news, highlights, exclusive interviews and in-depth features about her fashion endeavors, personal life and charitable efforts.
Frankger
| #
Biography of American professional gervonta-davis-az.com boxer Gervonta Davis: career highlights, weight, records, famous fights, personal life, controversies and latest 2024 updates.
Leroynop
| #
Discover the world of Brad Pitt brad pitt az com with our dedicated website, offering extensive coverage of his illustrious career, upcoming projects, and personal endeavors.
WalterBorne
| #
how to book a tesla rent a electric car near me
GregoryUniog
| #
У нас вы можете купить айфоны https://vk.com/crazy_humor01 оптом по самым лучшим ценам. Оригинальные смартфоны Apple с гарантией качества. Постоянное наличие популярных моделей.
JesusLaple
| #
Квартирный переезд https://spb-gruzoperevozka.ru с грузчиками быстро и качественно! Упакуем, вынесем, перевезем и разместим вещи на новом месте. Надежная команда, аккуратность и доступные тарифы.
Josephdof
| #
стол овальный офисный для переговоров https://mm26.ru
Abigael206
| #
You have done a amazing job with you website
Willierox
| #
Откройте для себя последние kraftsir.ru новости, аналитику и экспертные обзоры о спорте и ставках. Узнайте о лучших стратегиях ставок, следите за актуальными событиями в мире спорта и получите все необходимые инструменты для успешных ставок.
GermanHit
| #
киного фильмы про магию kinogo фильмы о космосе
Williamhit
| #
киного фильмы 2025 kinogo фильмы про оборотней
Davidinelt
| #
Подробные стратегии покера https://fatcurus.ru и анализ турниров: эффективные тактики, разбор раздач и ключевые советы для улучшения игры. Только практическая информация для выигрышей.
Calvinloash
| #
Новости игровой индустрии depcult35.ru/ аналитику и обзоры самых популярных игр. Читайте о новинках, трендах и получайте полезные советы для улучшения игрового процесса.
Romanspeab
| #
Все о мире гэмблинга https://minsvyazcc.ru обзоры казино, игры, стратегии и последние новости индустрии. Узнайте о новых слотах, бонусах и тенденциях в азартных играх.
JeffreyVep
| #
Все главные события https://tankdiv.ru мира спорта: обзоры турниров, результаты матчей, аналитику и актуальные новости из самых популярных дисциплин.
StephenAmimb
| #
снять проституток калуга шлюхи калуги
Jameszet
| #
OR Realty — это ваш надежный партнер в мире недвижимости. Мы предлагаем большой выбор квартир, домов и коммерческих объектов по выгодным условиям. Наши специалисты помогут вам найти идеальный вариант, соответствующий вашим потребностям. Надежность, качество и удобство — вот что делает OR Realty лучшим выбором. Обращайтесь!
RobertTaw
| #
скачать приложение mostbet mostbet 1-15
Williamcow
| #
1win скачать 1win aviator
DonaldDaymn
| #
Свежие новости спорта angryfoxtattoo откройте для себя последние спортивные новости, аналитику ставок и прогнозы экспертов на азербайджанском языке. Самая актуальная информация о футболе, киберспорте и других видах спорта здесь!
JamalPoexy
| #
mostbet live mostbet uzb
ForestSax
| #
увлекательные стратегии mexatrondiy ru репортажи с турниров и последние новости покера. Станьте мастером игры, окунитесь в мир азартных карт с нашим сайтом
Odelldralk
| #
узнайте последние новости umra tour ru киберспорта, анализ турниров и игровые стратегии в Азербайджане. Присоединяйтесь к нам, чтобы побеждать в мире киберспорта.
Gilbertseics
| #
Актуальные и свежие новости gastromoroz.ru спорта и казино. Узнайте актуальные спортивные события, анализы матчей, советы по ставкам, обзоры казино игр и стратегии.
KeithNep
| #
[url=https://katun-room.ru]Katun Room[/url] — это уютные гостиницы в самом сердце природы Алтая. Мы предлагаем комфортное проживание рядом с живописной рекой Катунь. Номера на любой вкус и бюджет, тишина и свежий воздух создадут незабываемую атмосферу для вашего [url=https://katun-room.ru]отдыха[/url]. Забронируйте ваш номер уже сегодня!
MichaelGem
| #
kinogo фильмы ужасов киного сериалы про преступников
AnthonyErork
| #
киного фильмы для ноутбука kinogo мультфильмы
Robertsok
| #
kinogo короткометражки kinogo фильмы по студиям
Jamesacuth
| #
A modern AI tool ai undress for working with images. Learn more about its features, capabilities and applications. Full privacy control and ease of use will ensure comfortable interaction.
LucasVuh
| #
Footballer Bukayo Saka’s biography http://bukayo-saka-az.org personal life, Nigerian heritage, religious views and girlfriend’s name. Salary, playing stats, jersey number, Euro appearances with England and matches with Arsenal. Latest news for 2024.
Elliotcenom
| #
Basketball player Jimmy Butler’s jimmy butler biography: personal life, Selena Gomez relationship rumors, height, cars, idol stats, Miami Heat salary, injury news and his coffee brand. Latest news of 2024.
ErnestGab
| #
Biography of footballer Joshua Kimmich jimmy butler personal life, wife and children. Career at Bayern Munich, statistics for Germany, work with Pep Guardiola, negotiations with Barcelona. Vice-captain at Euro 2024. Latest news for 2024.
TylerGam
| #
Djokovic’s tennis career novak-djokovic-az.org/ has been marked by numerous Grand Slam titles, showcasing his style of play. He has been a constant competitor to Rafael Nadal and Roger Federer.
StevenVor
| #
Свежие футбольные новости vseofootball.ru/ обзоры матчей, Лига чемпионов, статистика и лучшие букмекерские бонусы для ставок на спорт.
JamesMic
| #
Откройте мир мобильных игр http://games-ru.ru рейтинги лучших проектов, тренды, советы и гайды. Играйте в популярные шутеры, RPG, стратегии и песочницы прямо на телефоне!
NormanniG
| #
Все о Тони Кроосе http://toni-kroos.ru на одном сайте: биография, актуальные новости, детальная статистика и эксклюзивные обновления о немецкой футбольной звезде. Присоединяйтесь к сообществу фанатов и будьте в курсе всех событий, связанных с Кроосом!
BradyShoft
| #
Источник новостей о футболе futbol-vpered сборной России, Кубке России, Лиге Европы и лучших букмекерских предложениях. Узнайте последние результаты, прогнозы и аналитику!
Williekig
| #
Погрузитесь в захватывающий мир cleopatra-slot ru Cleopatra Slot! Узнайте о правилах игры, бонусных функциях и стратегиях, чтобы увеличить свои шансы на выигрыш.
Kennethbeend
| #
Все о ставках на спорт betting-ru.ru лучшие стратегии, прогнозы экспертов, обзор букмекерских контор и советы для успешного беттинга. Узнайте, как заработать на спортивных ставках!
RussellKab
| #
Исследуйте Zeus vs Hades zeus-vs-hades-download ru Gods of War! Играйте бесплатно, получайте фриспины и узнайте все о слоте 2024 года!
CharlesReula
| #
In Jodo Do Tigrinho online slots, every spin is a step towards victory! Incredible graphics, exciting themes and many bonuses await you. Fortune favors the brave – try your hand and discover the world of winnings with Tigrinho!
Richardmum
| #
Cryptocurrency trading service bitqt with AI is automation and efficiency. Artificial intelligence monitors market dynamics, reduces risks and optimizes transactions. The perfect solution for beginners and professionals.
Metamask Wallet
| #
MetaMask Download was a breeze! Setting it up on Safari and Opera was equally simple. If you’re starting out, https://metanaito.net/ is a great resource.
Graphic Designer
| #
Funny t-shirts for sale offer a great way to add personality and humor to your wardrobe. As a t-shirt graphic designer, I love creating designs that make people laugh, whether it’s through clever wordplay, quirky illustrations, or pop culture references. Each design is an opportunity to turn a simple shirt into a fun statement piece that brightens someone’s day. It’s rewarding to know that a little creativity and humor can make fashion more enjoyable for others!
https://comiko.net/u/2432768-tshirtsale
GenaroAgelt
| #
Откройте для себя Mega Joker mega-joker.ru/ увлекательный видеослот от NetEnt, который сочетает в себе ностальгическую атмосферу классических автоматов и современные возможности выигрыша.
StanleyVoism
| #
Откройте для себя мир starlight princess slot Starlight Princess Slot! На нашем сайте вы найдете свежие новости, стратегии для увеличения выигрышей и эксклюзивные бонусы.
Aarondorgo
| #
Погрузитесь в мир Plinko plinko-ru.ru увлекательной игры, основанной на случайности и стратегии! Узнайте правила, механики и стратегии для успешной игры, чтобы увеличить свои шансы на крупные выигрыши.
Patricklam
| #
Aviatrix game https://aviatrix-games.com/en/ has become a sensation in the world of crash games. Its unique format, featuring a rapidly growing multiplier and the possibility of an unexpected crash. Aviatrix crash game is at 1win, 1xbet, Mostbet, and Pin Up.
Donaldmesty
| #
Захватывающий слот Lucky Jet lucky-jet-play-crash.ru/ от Spribe с уникальной краш-механикой, который предлагает игрокам шанс испытать свою удачу и стратегическое мышление.
Patricklam
| #
Aviatrix game https://aviatrix-games.com/en/ has become a sensation in the world of crash games. Its unique format, featuring a rapidly growing multiplier and the possibility of an unexpected crash. Aviatrix crash game is at 1win, 1xbet, Mostbet, and Pin Up.
GeorgeFut
| #
Откройте для себя водное поло water-polo-ru.ru/ историю, правила, тактики и влияние этого захватывающего водного вида спорта. Узнайте, как динамика и стратегия сочетаются, делая водное поло уникальным и увлекательным.
KevinPef
| #
Добро пожаловать на сайт redtigerplay.ru посвященный слотам Red Tiger! Узнайте последние новости, стратегии для увеличения выигрышей и эксклюзивные бонусы.
DennisSlilt
| #
Погрузитесь в захватывающий мир баккары http://baccarat-ru.ru узнайте историю игры, её правила, стратегии и секреты успеха. Откройте для себя элегантность и интригу одной из самых престижных карточных игр казино.
KerryFum
| #
Откройте для себя мир гандбола https://handball-ru.ru изучите историю игры, основные правила, ключевые стратегии и влияние на культуру.
CliftonTAH
| #
Погрузитесь в увлекательный мир покера http://poker-ru.ru исследуйте его историю, правила, продвинутые стратегии и культурное влияние. Узнайте, как интеллект и интуиция объединяются в этом захватывающем карточном спорте.
KeithPreag
| #
Мир блэкджека http://blackjack-ru.ru узнайте историю игры, её правила, секретные стратегии и влияние на современную культуру. Откройте для себя глубину этой захватывающей карточной игры и научитесь побеждать с умом.
Aaronoreva
| #
Погрузитесь в мир фигурного катания figure skating исследуйте его историю, уникальные техники, знаменитых спортсменов и культурное влияние.
ArmandoCrype
| #
Скачайте бесплатно книгу https://storitelling.ru по сторителлингу и узнайте, как создавать истории, которые цепляют с первых строк. Практические советы, примеры и вдохновение для всех, кто хочет освоить искусство рассказчика.
Albertjeamb
| #
Сайт игровых промокодов csgo500 промокод это ваш доступ к эксклюзивным бонусам и скидкам. Бесплатные награды, внутриигровая валюта и уникальные акции ждут вас. Успейте воспользоваться всеми возможностями!
PatrickUtibe
| #
Хотите раскрутить раскрутить телеграм-канал мы знаем, как это сделать! Поможем увеличить охваты, привлечь активную аудиторию и вывести ваш контент на новый уровень.
ThomasSTOVE
| #
вызвать капельницу от запоя на дому http://www.medlinks.ru/article.php?sid=110769
Arminda Bombino
| #
I close my browser often for various reasons, and having to repeatedly log into my accounts like Facebook, Yahoo, Gmail, and etc, is very inconvenient and getting gold. Firefox 2 never did this, just my current Firefox 3..
MatthewZit
| #
Ищете промокоды для игр rust4real промокоды на кейсы наш сайт – ваш лучший помощник! Собираем актуальные игровые промокоды для бонусов, скидок и эксклюзивных наград.
WesleyTooxy
| #
Участок в Мишкином Лугу http://мишкинлуг.рф/uchastki-mishkinlug по Симферопольскому шоссе — идеальное место для строительства! Тихий поселок, прекрасные виды, удобный подъезд и все условия для комфортной жизни.
MitchSlest
| #
glory-casino-bd.online
ShawnRubre
| #
Флешка оптом криптекс и купить ручку с флешкой в Новороссийске https://flashki-optom-1.ru/
MelvinEtelf
| #
Отзывы о компаниях и работодателях https://potrebsojuz.ru в одном месте. Узнайте реальное мнение сотрудников и клиентов, чтобы принять правильное решение при выборе работы или услуг.
EdwardAvaix
| #
Комплексная юридическая помощь https://kramzenergo.ru для вас и вашего бизнеса. Анализ дел, представительство в судах, поддержка на всех этапах
Stevensainc
| #
https://odnazhdyvskazke-tv.ru/
Frankpef
| #
вывод из запоя капельница на дому http://www.medlinks.ru/article.php?sid=110769
Howardven
| #
1win argentina 1win sign in
ThomasCah
| #
1win bono sin deposito app 1win
Arthurkes
| #
Откройте для себя историю слово пацана кровь на асфальте честный взгляд на суровую реальность, где дружба и слово дороже всего. Уникальный проект о жизни без прикрас и ценности принципов.
Metamask Chrome
| #
Secure your cryptocurrency by setting up your MetaMask Wallet today. For helpful tips, visit https://kingroada.com/.
JamesVer
| #
kinogo лучшие фильмы по комиксам киного российские сериалы
Richardanype
| #
киного фильмы по режиссерам киного сериалы по алфавиту
AlvinRut
| #
Vavada Casino to miejsce, gdzie emocje gry są zawsze na wyciągnięcie ręki. Oferujemy bogaty wybór najlepszych automatów do gier, klasycznych gier stołowych, takich jak poker, blackjack czy bakarat, a także wciągającą ruletkę na żywo z udziałem profesjonalnych krupierów. Nasza platforma Vavada Casino jest w pełni licencjonowana i zgodna z obowiązującymi przepisami prawa, co zapewnia bezpieczne i legalne środowisko rozgrywki dla wszystkich graczy.
CharlesAvefE
| #
kinogo приключения kinogo авторское кино
StevenAmori
| #
Witaj w Slottica PL! To miejsce, gdzie ekscytacja spotyka się z różnorodnością, a niezawodność staje się Twoim codziennym towarzyszem w świecie gier online. Dla polskich graczy w Slottica Casino przygotowaliśmy ofertę, która spełni wszystkie oczekiwania – od doskonałej kompatybilności mobilnej, przez szeroką gamę gier, aż po usługi skoncentrowane na Twoim komforcie i bezpieczeństwie.
DavidOneks
| #
Откройте для себя блэкспрут возможности даркнет-рынка с тысячами предложений. Быстрая регистрация, надежные сделки и анонимность на каждом этапе.
TimothyRox
| #
Лучшее онлайн казино https://1wincasino.pl Огромный выбор автоматов, настольных игр и live-казино. Уникальные акции, приветственные бонусы и мгновенные выплаты сделают вашу игру еще интереснее.
BradleyOptof
| #
веселая песня текст https://faav.ru
Metamask Chrome
| #
The Metamask extension has truly revolutionized how I manage my crypto wallets. If you’re looking to securely handle your funds, definitely check out https://metamenu.org/ for an easy guide!
JosephAcign
| #
охота на медведя луки для охоты
Jamesfef
| #
Полный комплекс услуг по оформлению медицинской лицензии с гарантией за 14 дней по договору. Детали: https://www.gta5-mods.com/users/MDLicenzii
WilliamKar
| #
Ресторанный консалтинг UPSKILL https://upskilll.ru/consulting это профессиональные услуги в сфере ресторанного бизнеса: анализ рынка, аудит заведения, устранение слабых мест, обучение персонала, занимаемся наставничеством рестораторов с 2018 года.
Washington post
| #
Washington post Surreal meaning.
Amphibia. Map of tennessee. Abrasion. Kitty.
JasonAlorm
| #
недорогой ремонт стиральных машин на дому ремонт стиральных машин bosch подольск
Roberthor
| #
Платформа Курьер Купер https://cash-kuper.ru открывает возможности для работы в доставке. Свободный график, прозрачная система оплаты и заказы поблизости – идеальный выбор для тех, кто ищет подработку или основной заработок.
StanleyLex
| #
cannabis delivery in prague thc gummies in prague
TommyPal
| #
cannabis for sale in prague https://shop-cannabis-prague.com
Charlesblege
| #
ремонт бойлера цена ремонт накопительных водонагревателей
CliffDem
| #
взять займ онлайн микрозайм
Thomasgeorm
| #
Открывайте кейсы CS:GO https://www.facebook.com/people/Hotdrop_CSGO/61550490187523/ с крутыми шансами на редкие скины. Удобный интерфейс, надежная система и огромный выбор кейсов сделают игру еще интереснее. Начните свой путь к топовым скинам прямо сейчас!
Josephkenue
| #
Bukmacher oraz kasyno internetowe Mostbet PL to jeden z najdłużej działających serwisów hazardowych w sieci z ofertą dla Polaków, gdyż został założony jeszcze w 2009 roku. Platforma może pochwalić się posiadaniem aktywnej licencji od Curacao, która gwarantuje bezpieczeństwo. Gracze w Mostbet Casino będą mogli spotkać tutaj ponad 3000 zróżnicowanych gier hazardowych, które zostały podzielone na automaty, gry stołowe oraz gry na żywo.
MarvinSyday
| #
Откройте яркие кейсы CS:GO кс кейсы и получите шанс выиграть топовые скины! Широкий выбор кейсов, высокий шанс дропа и честная система обеспечат увлекательный опыт.
Richardroope
| #
Пробуйте удачу https://discord.com/invite/B5fF6pW8Cm CS:GO! Шанс получить редкие и дорогие скины, широкий выбор кейсов и удобный интерфейс делают процесс открытия легким и захватывающим.
Carlogal
| #
Рискни и испытай удачу https://t.me/hotdropcases Шанс получить редкие и дорогие скины, широкий выбор кейсов и удобный интерфейс делают процесс открытия легким и захватывающим.
Aaronfance
| #
Ваши любимые кейсы CS:GO https://t.me/s/hotdropcases в одном месте! Большой выбор, удобный интерфейс и высокая вероятность выпадения редких предметов делают процесс открытия кейсов по-настоящему захватывающим.
Oswaldoskync
| #
Сервис бытовых услуг https://gidrostok-servis.ru это удобное решение для любых домашних задач. Уборка, ремонт, сантехника, установка техники и многое другое. Надежные специалисты, быстрое выполнение и доступные цены!
EdwardBelty
| #
подборка фильмов на вечер в хорошем качестве новинки кино 2025 без регистрации
ForestEndab
| #
Нужны деньги срочно взять микрозайм онлайн с быстрым одобрением и моментальным переводом на карту. Минимум документов, удобные условия и прозрачные ставки. Оформите займ прямо сейчас!
Michaelpoole
| #
новые подборка фильмов на вечер 2025 лучшие смотреть кино бесплатно фильмы
Thomasfaimi
| #
Промокоды для игр https://esportpromo.com/cs2/ это бесплатные бонусы, скидки и эксклюзивные награды! Находите актуальные коды, используйте их и получайте максимум удовольствия от игры без лишних затрат.
JamesNot
| #
Лучшие игровые промокоды промокоды standoff 2 на кейсы в одном месте! Активируйте бонусы, получайте подарки и прокачивайте аккаунт без лишних затрат. Следите за обновлениями, чтобы не пропустить новые промо!
Williamfek
| #
Лучшие игровые промокоды промокоды на барабан бонусов ggstandoff в одном месте! Активируйте бонусы, получайте подарки и прокачивайте аккаунт без лишних затрат. Следите за обновлениями, чтобы не пропустить новые промо!
Roberttiree
| #
Оптовые продажи сувениров и подарков и нанесение сувенир оптом в Кемерово: подарки с Нанесением логотипа компании оптом
Andrewabawl
| #
Бесплатные промокоды https://playpromocode.com/standoff/bulldrop/ для ваших любимых игр! Получайте монеты, бустеры, скины и другие ценные награды. Мы собираем только проверенные коды и обновляем их каждый день.
Richardbaina
| #
Хотите проверить компанию https://innproverka.ru по ИНН? Наш сервис поможет узнать подробную информацию о юридических лицах и ИП: статус, финансы, руководителей и возможные риски. Защищайте себя от ненадежных партнеров!
Rickyrouch
| #
Недвижимость на Северном Кипре https://iberiaproperty.ru выгодные инвестиции и комфортная жизнь у моря. Апартаменты, виллы и пентхаусы по доступным ценам. Поможем выбрать лучший вариант и оформить покупку.
Michaelfoege
| #
удобный маркетплейс bs2best с высоким уровнем анонимности и надежной системой защиты. Интуитивный интерфейс, проверенные продавцы и безопасные сделки делают его лучшим выбором для покупок.
Davidfah
| #
Раскрутка в соцсетях https://nakrytka.com без лишних затрат! Привлекаем реальную аудиторию, повышаем охваты и активность. Эффективные инструменты для роста вашего бренда.
Kevintut
| #
увлекательный сериал https://odnazhdy-v-skazketv.ru о жизни сказочных персонажей в реальном мире. Интригующий сюжет, волшебные события и неожиданные тайны. Смотрите онлайн в высоком качестве прямо сейчас!
Rubenesofe
| #
оказание услуг железнодорожных перевозок грузов https://bvs-logistica.com/zheleznodorozhnye-perevozki-gruzov.html
DavidToure
| #
Интернет-магазин товаров https://vitasleep.ru для здорового сна. В ассортименте: ортопедические матрасы, подушки, одеяла, постельное белье и аксессуары от проверенных брендов. Удобный выбор, доставка по России, гарантия качества. Забота о вашем комфорте и здоровом сне!
Brianmaige
| #
escort paris
WilliamVerge
| #
лучшие сериалы онлайн https://lordseriall.org
Percyjix
| #
смотреть сериал все серии подряд сериалы смотреть
BruceBEs
| #
сериалы онлайн подряд смотреть сериал
Josephchoks
| #
смотреть сериалы подряд https://lordserial7.com
JeffreyCak
| #
смотреть сериалы в хорошем качестве https://lordserials2.net
Michaelarguh
| #
смотреть сериал хороший без рекламы https://lordserials.cc
MetaMask Chrome
| #
If you’re new to crypto and need a guide on how to install the Metamask extension, check out https://sites.google.com/view/metamask-extension-dfkasdkfdnt/download. It walks you through everything in a simple way, making it easy to get started. I highly recommend their content!
StevenNot
| #
https://gruzoperevozki-minsk24.ru/
TravisNal
| #
Логистические услуги в Москве https://bvs-logistica.com доставка, хранение, грузоперевозки. Надежные решения для бизнеса и частных клиентов. Оптимизация маршрутов, складские услуги и полный контроль на всех этапах.
ThomasHam
| #
смотреть сериалы подряд https://lordserialss.life
Chrispen
| #
сериалы 2022 смотреть онлайн https://lordsserial.xyz
StephenKef
| #
Хотите почувствовать азарт? arkada casino бонусы за регистрацию предлагает широкий выбор игр, честную систему выигрышей и мгновенные выплаты. Захватывающие слоты и бонусы ждут вас!
GeorgeRog
| #
смотреть лучшие сериалы https://lordseriall6.org
Georgewah
| #
смотреть сериал регистрация https://lordsserials.org
Jamescully
| #
лучшие сериалы онлайн https://lordserial5.pet
Andrewprund
| #
Check out Sellvia https://www.instagram.com/sellvia.dropshipping/ on Instagram for the hottest product ideas, store upgrades, and exclusive deals! Stay in the loop with our latest dropshipping tips and grab promo coupons to boost your business.
GerardFum
| #
Где заказать монтаж плоской кровли с битумной гидроизоляцией, нужны контакты – https://ploskaya-krovli-zagorodnyh-domov.ru/
MetaMask Chrome
| #
I’ve been looking for a reliable way to install Metamask on Chrome, and https://sites.google.com/view/metamask-extension-download-oa/chrome provided exactly what I needed. Their guide made the process effortless.
Numbersbeica
| #
Ищете выгодные курсы? Актуальная подборка со скидками до 99%. Перейти к выбору https://www.intensedebate.com/people/Sofia93
Jeffryexpax
| #
The full special bip39 Word List consists of 2048 words used to protect cryptocurrency wallets. Allows you to create backups and restore access to digital assets. Check out the full list.
Robertzep
| #
Reliable and unique bip39 Word List contains 2048 words needed to create seed phrases in crypto wallets. Allows you to safely manage private keys and guarantees the possibility of recovering funds.
Robertzep
| #
Reliable and unique bip39 Word List contains 2048 words needed to create seed phrases in crypto wallets. Allows you to safely manage private keys and guarantees the possibility of recovering funds.
Michaelblova
| #
cannabis shop in prague https://sale-weed-prague.com
Jerrydag
| #
1xBet promo code https://space-find.net/2024/10/22/step-by-step-guide-to-using-the-1xbet-new-account/ your chance to get bonuses on bets, free bets and exclusive promotions! Enter the code during registration and start playing with an increased deposit.
BradleyUnlic
| #
Ремонт компьютеров и ноутбуков https://remcomp89.ru в Новом Уренгое – быстрые и качественные услуги! Диагностика, настройка, замена комплектующих, восстановление данных. Гарантия на работу, доступные цены и выезд мастера!
arkada casino автоматы
| #
Играйте в аркадные игры в лучшем онлайн казино | Играйте в аркадные игры и выигрывайте крупные призы | Увлекательные аркадные игры и безграничный азарт | Играйте в аркады и выигрывайте деньги | Играйте в увлекательные аркады и выигрывайте призы | Ощутите драйв аркад в онлайн казино | Выигрывайте крупные суммы в аркадах | Самые популярные аркадные игры в одном казино | Лучшие аркадные развлечения для вас | Выигрывайте крупные денежные призы в аркадах | Большие денежные призы в аркадах | Побеждайте в аркадных баталиях и зарабатывайте деньги | Побеждайте в аркадах и станьте миллионером | Играйте в аркады и получайте удовольствие | Побеждайте в аркадах и получайте денежные призы | Побеждайте в аркадных играх и получайте призы
arkada регистрация arkada casino online .
JosephGaw
| #
Looking for the current spinbetter promo code? Get bonus funds for bets and casino games. Easy activation, favorable conditions and real winnings are waiting for you. Hurry to use it!
HaroldMon
| #
Используйте актуальный промокод 1xbet и получите увеличенный бонус на первый депозит! Делайте ставки на спорт, играйте в казино и пользуйтесь эксклюзивными предложениями. Легкая регистрация и моментальные выплаты!
заказать входную дверь
| #
Секреты успешной покупки входной металлической двери, которая прослужит долгие годы.
Лучшие магазины с широким выбором входных металлических дверей.
дверь металлическая входная цена входная дверь .
Что учесть при выборе входной металлической двери.
5 основных причин купить металлическую входную дверь.
В чем отличия входных металлических дверей разных производителей.
заказать двери входные металлические
| #
Как выбрать идеальную входную металлическую дверь, соответствует всем требованиям безопасности.
Где купить надежную входную металлическую дверь по выгодной цене.
заказать входную дверь дверь металлическая входная .
Как не ошибиться с выбором входной металлической двери.
5 основных причин купить металлическую входную дверь.
Как выбрать между дверью одного бренда и дверью другого.
бонус код arkada casino
| #
Онлайн казино с широким выбором аркадных игр | Погрузитесь в мир азарта и аркадных развлечений | Увлекательные аркадные игры и безграничный азарт | Азартные аркады и огромные выигрыши | Лучшие аркадные игры в онлайн казино | Уникальные аркады только у нас | Погрузитесь в мир аркадных развлечений и азарта | Азарт и аркады в одном месте | Играйте в аркадные игры и ощутите азарт | Аркадные игры и азарт ждут вас в онлайн казино | Аркадные игры для всех желающих азарта | Выигрывайте крупные суммы в аркадах | Испытайте свою удачу в аркадном казино | Играйте в аркады и получайте удовольствие | Выигрывайте крупные суммы в аркадных играх | Увлекательные аркады и возможность заработать деньги
arkada casino официальный аркада регистрация .
Steventeaby
| #
bip39
Andrewquick
| #
The most comprehensive bip39 for securely creating and restoring cryptocurrency wallets. Learn how mnemonic coding works and protect your digital assets!
Everetteremy
| #
moving cost estimate Prague Prague to Vienna transport
жалюзи на окна автоматика
| #
Современная автоматика для жалюзи, повысит комфорт в помещении.
Снижайте затраты на отопление и кондиционирование воздуха с автоматизацией жалюзи.
Контролируйте жалюзи с помощью смартфона.
Создайте уютную атмосферу с автоматическими жалюзи.
Качественное обслуживание автоматизированных жалюзи.
автоматика на горизонтальные жалюзи автоматика на горизонтальные жалюзи .
Williamder
| #
Ищете квартиру в новом доме? новостройки Украина – это современные жилые комплексы, доступные цены, рассрочка и ипотека. Подберите идеальное жилье от надежных застройщиков!
Brettcib
| #
Останні актуальні останні новини україна оперативні події, важливі рішення, міжнародна політика та економіка. Все, що потрібно знати про життя країни, в одному місці!
Richardtraft
| #
Продажа инструментов для дома https://profimaster58.ru строительства и ремонта! Огромный выбор ручного и электроинструмента, выгодные цены, акции и быстрая доставка. Найдите все необходимое в одном месте!
BradleyPhoks
| #
Free and reliable best nudify ai tool to create images with AI? The Best Free AI Nudify is the best choice for creating nude photos with privacy and quality guarantee.
Kennethgeore
| #
грузчики челябинск газель недорого услуги профессиональных грузчиков
bip39
| #
Use the proven bip39 phrase standard to protect your assets and easily restore access to your finances. A complete list of 2048 mnemonic words used to generate and restore cryptocurrency wallets.
Raymondmek
| #
Sitio web oficial jude-bellingham.com mx de fans de Jude Bellingham: noticias, logros y material exclusivo sobre la carrera del talentoso mediocampista que juega en el Real Madrid.
rodri
| #
Sitio web de fans https://rodri.com.mx de Rodri Hernandez: Descubre la carrera y logros del mediocampista espanol del Manchester City. Noticias, estadisticas y analisis del juego de uno de los mejores futbolistas actuales.
wjkawsdas_rot
| #
Desde 2019, a Mostbet Portugal tem se consolidado como uma excelente escolha para os jogadores portugueses que buscam formas seguras e divertidas de apostar e jogar nos casinos online e ao vivo. Com uma ampla seleção de jogos, apostas esportivas, esports e apostas em esportes virtuais, a plataforma oferece um catálogo premium de uma marca licenciada e confiável. Confiança, inteligência e lealdade são os pilares que sustentam a Mostbet Casino, que ainda proporciona bônus e promoções exclusivas, métodos de pagamento locais reconhecidos e ferramentas para um jogo responsável: Mostbet Casino
RalphNuast
| #
Kevin De Bruyne kevin-de-bruyne.com mx es un maestro del futbol moderno, conocido por su vision de juego, precision y liderazgo en el Manchester City. Su talento y trabajo duro lo han convertido en una leyenda del deporte.
Mathewfam
| #
Sitio fan de Mohamed Salah mohamed salah com mx ultimas noticias, records, entrevistas y los mejores momentos de la carrera de uno de los futbolistas mas grandes de la actualidad. ?Mantente al tanto!
UlyssesBef
| #
Fan site de Vinicius Junior https://vinicius-jr.com.mx noticias, logros y detalles sobre su carrera en el Real Madrid. Sigue la evolucion de esta estrella del futbol mundial.
Cecilacido
| #
application casino en ligne casino en ligne francais
NormanDwele
| #
Try your luck at pinup casino! The best slots, roulette, blackjack and live games with real dealers. Pleasant bonuses, promotions and a user-friendly interface will create ideal conditions for the game!
email4domain
| #
Почта для домена на https://email4domain.ru это корпоративный почтовый сервис email для организаций. Почта для домена обеспечивает сотрудников адресами электронной почты на собственном домене компании. Почта для бизнеса улучшает узнаваемость и повышает имидж бренда.
жалюзи с приводом на окна
| #
Лучший выбор для автоматизации жалюзи.
Умное устройство для управления жалюзи.
для вашего дома.
Зачем нужен привод для автоматизации жалюзей.
Тенденции в автоматизации жалюзи с помощью привода.
Как управлять жалюзями с помощью смартфона с использованием привода.
Шаг за шагом по инструкции по установке привода для жалюзи.
Экономьте время и энергию с автоматизированным приводом для жалюзи.
Интересные факты о приводах для жалюзи.
Как повысить уровень комфорта с приводом для жалюзи.
привод жалюзи электрический привод жалюзи электрический .
Jamessit
| #
Full wordlist New full BIP39 2048 words used to create and restore crypto wallets. Multi-language support, high security and ease of use to protect your funds. 2048 mnemonic words for seed generation.
KeithDUg
| #
New full Bip39 2048 words used to create and restore crypto wallets. Multi-language support, high security and ease of use to protect your funds.
Franknoump
| #
casino en ligne francai https://annuaire-des-casinos.com
Franknoump
| #
casino en ligne fiable forum arlequin casino en ligne
Jamessit
| #
Full wordlist New full BIP39 2048 words used to create and restore crypto wallets. Multi-language support, high security and ease of use to protect your funds. 2048 mnemonic words for seed generation.
GlennElilk
| #
Актуальные промокоды казино https://akivaschool.com/pgs/code_promotionnel_1xbet_depot.html получите дополнительные бонусы при регистрации! Увеличенный первый депозит, бесплатные ставки, фриспины и эксклюзивные акции для игроков. Вводите код и выигрывайте больше!
KennethEffex
| #
Промокод казино https://uconnect.ae/read-blog/190468 активируйте эксклюзивные бонусы! Получите фриспины, бонусные деньги на депозит и кешбэк. Используйте актуальные коды для максимальной выгоды в онлайн-казино. Играйте с лучшими условиями!
DavidFed
| #
Current casino promo codes http://rzyuhua.com/tpl/inc/?1xbet_promo_code_welcome_bonus_free.html bonus money, free spins and cashback for playing. Use verified codes, activate exclusive offers and win more!
Jeffreyitady
| #
Ищете выгодные покупки? нова маркетплейс предлагает широкий выбор товаров по привлекательным ценам! Безопасные сделки, удобный интерфейс и проверенные продавцы – все для комфортного шопинга.
ThomasJap
| #
Промокод казино https://hyd-parts.ru/news/sovety_po_prohoghdeniyu_mage_knight_apocalypse_osnovnye_missii_086__2.html активируйте эксклюзивные бонусы! Получите фриспины, дополнительные деньги на депозит и кешбэк. Используйте актуальные коды для максимальной выгоды в онлайн-казино и увеличьте свои шансы на выигрыш!
Charlesonepe
| #
Лучшие промокоды для казино http://pharaon.mega-f.ru/logs/pros/kak_zapoluchity_bezuprechnuyu_koghu.html активируйте бонусные предложения и получите больше шансов на выигрыш. Бесплатные спины, дополнительные деньги на баланс и персональные акции ждут вас!
Jerryenums
| #
Присоединяйтесь к NovaXLtd.live https://novaxltd.live платформе с широкими возможностями и удобными инструментами. Доступность, надежность, поддержка!
Peterham
| #
Используйте Kra020 https://kra020.com надежная площадка с удобными инструментами и поддержкой 24/7. Простота и безопасность в одном сервисе!
DavidPer
| #
Откройте новые возможности https://sindicate24-4.biz! Удобный сервис, быстрые решения и выгодные предложения для пользователей.
Robertoweill
| #
דיסקרטיות בחיפה. תורות יהודיות מסורתיות מדגישות את חשיבות הצניעות והטוהר המיני של דירות דיסקרטיות בתל אביב, תוך חגיגות סופית נשוי או פרופסור מהטכניון – כאן כולם אורחים רצויים. כאן מקבלים פינוק שעושה טוב לכולם, והכל בדיסקרטיות מלאה. מבלים כאן חילונים שירותי פינוקים חיפה – טיפים מקצועיים לבחירת הבילוי האולטימטיבי בעיר
Ralphprulk
| #
Продажа сигарет mond сигареты купить в интернет-магазине – оригинальные бренды, доступные цены, удобные способы оплаты и быстрая доставка. Сделайте заказ сегодня!
StevenNit
| #
Откройте все возможности Blacksprut Большой выбор товаров, безопасные сделки и простая навигация. Приватность и защита пользователей на первом месте.
MetaMask Download
| #
If you want to safely install Metamask on Chrome, visit https://metanate.org/. Their guide is super detailed, making it easy to follow. Now I can manage my crypto without any worries.
лучшие решения для сцены с электрокарнизом
| #
Ваша сцена станет неповторимой с электрокарнизами, сделают ваше выступление незабываемым.
Электрокарнизы придают шоу утонченность, делая представление более динамичным.
Современные технологии в электрокарнизах, дарят возможность воплотить любую идею.
С электрокарнизами публика останется в восторге, с идеальным сочетанием функциональности и эстетики.
Электрокарнизы – современное решение для профессиональных выступлений, покоривших сердца зрителей.
Инновационные электрокарнизы для сцены, сделать ваше выступление неповторимым.
Уникальные решения для каждого типа представления, с легкой и тихой работой в каждом представлении.
Осуществите свои идеи с помощью электрокарнизов, и подчеркнуть важность каждой сцены.
Электрокарнизы – современное решение для сцены, и подчеркивая профессионализм исполнителей.
Выберите электрокарнизы по своему вкусу, обеспечив себе идеальный результат в каждом выступлении.
автоматический карниз для сцены автоматический карниз для сцены .
Jamesstype
| #
Свежие промокоды казино http://ihvo.de/wp-content/pages/1xbet_promo_codes_bonus.html бонусы, фриспины и эксклюзивные акции от топовых платформ! Найдите лучшие предложения, активируйте код и выигрывайте больше.
seo-base
| #
частный сео оптимизатор https://seo-base.ru
Waynesaf
| #
Нужен опытный лечебный массаж Ивантеевка? Помогаю при остеохондрозе, грыжах, искривлениях позвоночника и болях в суставах. Безопасные техники, профессиональный подход, запись на прием!
EdwinLiaiz
| #
Защитите авто от коррозии https://antikorauto.ru антикоррозийная обработка и кузовной ремонт с гарантией. Восстановление геометрии кузова, покраска, удаление вмятин. Долговечность и качество по доступным ценам!
vasya-muflonov
| #
Жарким летним утром Вася Муфлонов, заядлый рыбак из небольшой деревушки, собрал свои снасти и отправился на местное озеро. День обещал быть необычайно знойным, солнце уже в ранние часы нещадно припекало.
Вася расположился в укромном месте среди камышей, где, по его наблюдениям, всегда водилась крупная рыба. Забросив удочку, он погрузился в привычное для рыбака созерцательное состояние. Время текло медленно, поплавок лениво покачивался на водной глади, но рыба словно избегала его приманки.
Ближе к полудню, когда жара достигла своего пика, произошло то, чего Вася ждал все утро – поплавок резко ушёл под воду. После недолгой борьбы на берегу оказался огромный серебристый карп, какого Вася никогда раньше не ловил.
Радости рыбака не было предела, но огорчало одно – он не взял с собой фотоаппарат, чтобы запечатлеть этот исторический момент. Тут в голову Васи пришла необычная идея. Он аккуратно положил карпа себе на грудь и прилёг на песчаный берег под палящее солнце. “Пусть загар оставит память об этом улове”, – подумал находчивый рыбак.
Через час, когда Вася поднялся с песка, на его загорелой груди отчётливо виднелся светлый силуэт пойманной рыбы – точная копия его трофея. Теперь у него было неопровержимое доказательство его рыбацкой удачи.
История о необычном способе запечатлеть улов быстро разлетелась по деревне. С тех пор местные рыбаки в шутку стали называть этот метод “фотография по-муфлоновски”. А Вася, демонстрируя друзьям необычный загар, с улыбкой рассказывал о своём изобретательном решении.
Эта история стала местной легендой, и теперь каждое лето молодые рыбаки пытаются повторить трюк Васи Муфлонова, создавая на своих телах “загорелые фотографии” своих уловов. А сам Вася стал известен не только как умелый рыбак, но и как человек, способный найти нестандартное решение в любой ситуации.
gambling-soft
| #
white label casino solutions white label casino solutions
Shanesaw
| #
Fast and secure VPS https://evps.host/products for any task! Flexible configurations, SSD drives, DDoS protection. Easy scaling, high performance and 24/7 support!
Danielseila
| #
All about Vinicius Junior vinicius-junior-bd.com/ career, trophies, highlights and records. Follow the Brazilian winger, his success at Real Madrid and on the international stage!
WayneRAL
| #
All about Karim Benzema karim-benzema-bd com biography, achievements, main goals and career moments. Find out how the striker conquered Real Madrid and continues to shine in world football!
Timothyexerb
| #
All about Karim Benzema karim-benzema biography, achievements, main goals and career moments. Find out how the striker conquered Real Madrid and continues to shine in world football!
BasilSig
| #
https://sem-del.ru/
MetaMask Download
| #
A friend recommended I visit https://metamake.org/ for instructions on downloading Metamask, and I’m so glad I did! The guide was clear, and I had my wallet set up in no time. Definitely a must-visit site for crypto users!
WillieFar
| #
https://antimud.ru/
Scottper
| #
arkada casino online аркада casino официальный сайт
Michaeljog
| #
Военная служба по контракту https://contract116.ru стабильность, достойная зарплата, социальные льготы и карьерный рост. Присоединяйтесь к профессиональной армии и получите надежное будущее!
Chesterrit
| #
Kevin De Bruyne kevin de bruyne az com is the leader of the Manchester City and Belgium national team midfield. Find out all about his achievements, matches, awards and records in world football!
TommySip
| #
Joshua Kimmich https://joshua-kimmich-az.org the versatile leader of Bayern and the German national team! The latest news, stats, highlights, goals and assists. Follow the career of one of the best midfielders in the world!
DerekAluro
| #
скачать читы на самп аризона рп
mckeesportautobodylwag
| #
Transform the vehicle into a showstopper with expert car tuning services. At a shop, we provide a wide range of improvements to boost efficiency. Whether you want a unique appearance or top-tier motor adjustments, we’ve got you covered. Explore our offerings at https://mckeesportautobody.com/. Upgrade your ride today and experience excellence like never before.
Danielges
| #
Каталог подарков и сувениров https://kameshki51.ru/catalog/ из камня – эксклюзивные изделия из мрамора, гранита, оникса и яшмы. Шкатулки, статуэтки, часы, пресс-папье, бизнес-сувениры. Высокое качество и ручная работа!
ScottFak
| #
Подарки и сувениры из камня https://kameshki51.ru статуэтки, шкатулки, часы, панно, изделия из мрамора, гранита и оникса. Ручная работа, уникальный дизайн, доставка по России!
Michaelplast
| #
https://itscleaning.ru/
JosephFep
| #
Как побороть лудоманию https://teletype.in/@antosha-sevan/moi-opyt-izbavlenia-ot-igrovoi-zavasimosty история глазами прошедшего это испытание и несколько советам тем, кто в середине пути или столкнулся с этим через друзей и близких. Описываю в статье как Вавада помогла мне избавиться от игровой зависимости
CharlesSek
| #
Образовательный портал https://moi-slovari.ru собрание полезных материалов, лекций, статей и справочной информации для школьников, студентов и профессионалов. Учитесь легко и эффективно
JesseBroox
| #
https://cleanmsk799.ru/
Edwardkar
| #
Окунитесь в азарт с https://aviator-slot.ru Уникальная игра с захватывающим геймплеем и возможностью сорвать крупный выигрыш. Следите за коэффициентом, вовремя забирайте ставку и умножайте баланс. Испытайте удачу прямо сейчас!
BrianMunny
| #
Новости музыки Украины https://musicnews.com.ua популярные жанры, тренды и полезные советы для музыкантов. Узнавайте больше о мире музыки и развивайте свои навыки!
NormanImaph
| #
Ставки на спорт http://betting-ru.ru ваш шанс превратить знания в выигрыш! Лучшие коэффициенты, огромный выбор событий и удобный интерфейс. Делайте прогнозы и побеждайте!
MetaMask Download
| #
I wanted to download Metamask safely, and https://metaduck.org/ helped me do just that. Their guide is detailed and straightforward.
Williamsep
| #
сколько стоят услуги клининговой компании
MetaMask Download
| #
If you want to install Metamask on Chrome quickly and securely, check out https://metapaws.org/. The instructions are clear, making it perfect for beginners!
Raymondsag
| #
https://posle-remonta-uborka.ru/
ShawnFoups
| #
https://прочисто.рф/
Miltonseick
| #
Zeus vs Hades https://zeus-vs-hades-download.ru эпичная битва богов! Игровой автомат с грозными бонусами, фриспинами и множителями. Выберите сторону Зевса или Аида и сорвите большой выигрыш!
Kennethneirm
| #
Мир смешанных единоборств https://ufc-ru.ru (MMA) в UFC! Рейтинги, бои, истории бойцов, прогнозы на поединки и эксклюзивные материалы о крупнейшей бойцовской лиге.
JasonPap
| #
Все о легкой атлетике athletics-ru.ru История развития, основные дисциплины, рекорды, тренировки и современные тенденции. Узнайте больше о королеве спорта!
Augustglymn
| #
https://cleanliness-cleaning.ru/
JoshuaPef
| #
Cassino online Pin Up https://888casino-official-brazil.site ganhos sem limites! Jogue seus caca-niqueis favoritos, participe de torneios, ganhe cashback e rodadas gratis. Licenca, seguranca e pagamentos rapidos!
phoenixautobodyshopcoogy
| #
Revamp upgrade your ride with our premium superior car tuning services. We specialize in tailored modifications that elevate both capabilities and design. From horsepower boosts to exterior upgrades, we’ve got you covered. Experience the ultimate transformation at https://phoenix-autobodyshop.com/ and let our seasoned team bring your vision to life. Visit us today and drive with pride!
elon casino_byKi
| #
elonbet elonbet .
EdgarRox
| #
Свежие и актуальные новости https://sugar-news.com.ua Украины и мира! Будьте в курсе последних событий, аналитики и трендов. Читайте последние новости Украины онлайн!
bazovichok
| #
Лучшие эксперты в одном месте! реестр соут маркетплейс и агрегатор экспертных компаний помогает найти надежных специалистов по разным направлениям. Удобный выбор, проверенные отзывы и рейтинг!
DannyMinly
| #
ремонт квартир уборка
ArthuroxiVa
| #
https://clean-sharks.ru/
Free tanpa deposit
| #
Free tanpa deposit
A Brief History of Elemental Analysis » Artemis Analytical
elektrokar_yson
| #
Оптимизируйте свою жизнь с электрокарнизом с таймером, наслаждайтесь комфортом и современностью.
Создайте атмосферу уюта и стиля с помощью электрокарниза и таймера, сделает вашу жизнь проще и приятнее.
Освободите руки и ум с электрокарнизом и таймером, создайте идеальную атмосферу в вашем доме.
Оптимальное решение для автоматизации штор – электрокарниз с таймером, обеспечит вас и вашу семью уютом и функциональностью.
Инновационные технологии в управлении шторами – электрокарниз с таймером, добавит функциональности и удобства в вашу жизнь.
Электрический карниз с таймером https://prokarniz50.ru/ .
PatrickPhymn
| #
Хотите играть в Steam-игры shared-steam.org/ дешевле? Shared-Steam.org предлагает аренду аккаунтов с топовыми играми. Безопасность, доступность и удобство – ваш гейминг без границ!
nemeckoe porno_mool
| #
немки порно http://trax-nemeckoe1.ru/ .
americasbestcertifiedautobodycoogy
| #
Unleash enhance your vehicle’s full potential with our premium top-tier tuning services. We offer personalized modifications to improve your car’s performance, aesthetics, and comfort. Our expert certified technicians use state-of-the-art high-tech technology to deliver flawless outcomes. https://americasbestcertifiedautobody.com/ Whether you seek remarkable speed, handling, or luxury, we provide top-notch solutions. Trust us to elevate your driving experience. Contact us today and unveil the ultimate in automotive enhancement!
Davidcem
| #
Create images deep nude easily with AI. Remove clothes from anyone in the image and enjoy their nakedness.
slots-cool
| #
Turkiye’deki slot makineleri gercek parayla oyun slotlar gercek parayla oynanabilen slotlar kapsaml bir baks! Nerede oynan?r, hangi slotlar en karldr ve en iyi casino nasl secilir Kumar severler icin ipuclar, incelemeler, derecelendirmeler ve bonuslar!
slots-rait
| #
Turkiye Slotlar Rehberi Gercek parayla oyun slotlar En Iyi Slotlar ve Casinolar! Nerede oynayabileceginizi, hangi oyunlarn populer oldugunu ve bonuslar nasl alabileceginizi ogrenin. Oyun alanlar ve guncel eglencelere dair kapsaml bir genel baks!
MetaMask Download
| #
Thanks to https://download.metaredi.org/, I installed the Metamask extension without any trouble. Now I can manage my crypto easily and securely.
perevozimgruz_by
| #
перевозка велосипедов https://perevozimgruz.by/contacts/
perevozimgruz
| #
перевозка шкафа https://perevozimgruz.by
TerryTow
| #
Знакомства в Уфе https://ufavip.sbs с нашей платформой стали еще проще, можно встретить девушек, готовых к интересному общению и приятному времяпрепровождению. Здесь каждый найдет то, что ищет: от легкого флирта до серьезных отношений.
JamesRound
| #
https://uborka-kvartir-luberci.ru/
Josephhiz
| #
клининг уборка офиса
Edwardvam
| #
Is it possible to win at Lucky Jet? We analyze the main strategies, analyze player reviews, and give an honest assessment of the popular game. Read to avoid mistakes and increase your chances of success!
JacobPrurn
| #
https://klining-64.ru/
perevozimgruz_by
| #
квартирный переезд минск спб грузоперевозки минск
porno milfi_ahPi
| #
milf порно http://milfland-pro1.ru/ .
1win_ndkt
| #
ставки на спорт бишкек http://mabc.com.kg/ .
mostbet_sdSa
| #
mostbet вход в личный кабинет официальный http://gtrtt.com.kg .
porno mylt_hcEn
| #
порно мульты порно мульты .
porno ginekolog_xwkn
| #
порно с гинекологом порно с гинекологом .
умный электрокарниз
| #
Управляйте своим окружением с помощью программируемого электрокарниза, который легко устанавливается.
Трансформируйте ваше жилище с помощью программируемого электрокарниза, который дарит вам полный контроль.
Освежите свою обстановку с помощью программируемого электрокарниза, который позволит вам сохранить энергию.
Пусть ваша спальня станет местом для отдыха и релаксации с программируемым электрокарнизом, дарит вам возможность наслаждаться каждой минутой вашего сна.
Регулируйте интенсивность света и тепла с помощью программируемого электрокарниза, обеспечит вас уютом и спокойствием.
электрокарниз подключение к умному дому https://elektrokarniz190.ru/ .
Matthewjam
| #
Кружевной бюстгальтер https://www.wildberries.ru/catalog/117605496/detail.aspx без косточек с застежкой спереди — это идеальный выбор для тех, кто ценит комфорт и стиль. Изготовленный из мягкого и нежного кружева, он обеспечивает естественную поддержку, не ограничивая движения. Удобная застежка спереди позволяет легко надевать и снимать бюстгальтер, а его изысканный дизайн добавляет нотку романтики в ваш образ.
GeraldHom
| #
?El paraiso del juego en Pin Up Casino https://ganar-apuestas-deportivas.com te espera! Echa un vistazo a nuestra coleccion de tragamonedas, juegos en vivo y juegos de mesa. Bonos rentables, torneos y un programa VIP te proporcionaran el maximo de emociones. ?Empieza a jugar ahora y gana!
Georgevully
| #
Каталог финансовых организаций https://srochno-zaym-online.ru в которых можно получить срочные онлайн займы и кредиты не выходя из дома через интернет.
perevozimgruz
| #
грузоперевозки минск спб грузоперевозки по рб
srochno-zaym
| #
Каталог финансовых организаций srochno-zaym-online.ru в которых можно получить срочные онлайн займы и кредиты не выходя из дома.
Dannycet
| #
лучшие комедии 2025 hdrezka смотреть мелодрамы hd онлайн
perevozimgruzby
| #
грузоперевозки беларусь россия грузоперевозки минск
pochemuabold
| #
Почему зонтик был необходим Робинзону https://e-pochemuchka.ru/zontik-dlya-robinzona-neobhodimost-ili-izlishestvo/
elon casino_lqOa
| #
elon bet casino elon bet casino .
elon casino_abka
| #
bet elon bet elon .
elon casino_qyen
| #
elonbet casino http://www.elonbetting.com .
elon casino_hkSt
| #
elone casino elone casino .
1 vin_isEi
| #
1win login https://bbcc.com.kg/ .
srochnozaym
| #
Каталог финансовых организаций srochno-zaym-online.ru в которых можно получить срочные онлайн займы и кредиты не выходя из дома.
hdrezka
| #
новые трейлеры к фильмам 2025 hdrezka фантастика без рекламы на планшете
JamesOriew
| #
Форум для кулинаров https://food-recipe.ru и рестораторов! Лучшие рецепты, тренды гастрономии, обсуждения ресторанного бизнеса. Общайтесь, делитесь опытом и вдохновляйтесь новыми идеями. Присоединяйтесь к нашему кулинарному сообществу!
DallasHeice
| #
https://infoteka24.ru/2024/05/04/106105/
Blairletly
| #
Красивые поздравления https://photofile.ru в одном месте! Огромный выбор картинок и открыток для любого повода. День рождения, юбилей, профессиональные праздники – найдите идеальное поздравление!
StephenMub
| #
Если вы ищете профессионалов в сфере управления недвижимостью, обратитесь к этой компании, подробнее тут https://sketchfab.com/allianceprof
JamesFaf
| #
Новый уникальный маркетплейс NOVA, есть все! Москва, Питер, всегда свежие клады!Акция для новых магазинов – продай на 1 млн, получи 100к!Приглашаем к сотрудничеству рекламодателей!
RichardWaimb
| #
Del Mar Energy Inc
Larryseags
| #
бездепозитный бонус без пополнения бездепозит и фриспины при регистрации
Calvinrox
| #
hdrezka бесплатные фильмы hd rezka боевики новинки 1080p
Kennethsoisk
| #
Маркетплейс нового поколения https://x-nova.live уникальный сервис для удобных покупок и продаж. Интеллектуальный поиск, персонализированные рекомендации и безопасность сделок – все для вашего комфорта!
MetaMask Download
| #
If you’re looking for a step-by-step guide to install the Metamask extension, https://metamaker.org/#metamask-download is the perfect place. They explain everything in detail, ensuring you set up your wallet correctly and securely.
Larryter
| #
ключ доступа vpn
elektrokarnizi_jeki
| #
автоматические карнизы автоматические карнизы .
tk999_heer
| #
tk999 login tk999 login .
tk999_kymn
| #
tk999 tk999 .
KU9_gnkl
| #
KU9 bet KU9 bet .
kypit viagry_uqKa
| #
виагра для мужчин виагра для мужчин .
mtsnovosibirsksig
| #
мтс тв
https://ukskilledworkfinder.com/employer/job-daddy2052/
сайт мтс новосибирск
mikrozaymivah
| #
Получить кредит на банковскую карту теперь просто, моментально и комфортно. Мы обеспечиваем дружелюбные условия. Перейдите на https://mikro-zaymi.ru/, чтобы узнать все детали. Процесс оформления занимает несколько минут. Не задерживайтесь! Получите кредит прямо сейчас!
JosephWhopy
| #
Современный маркетплейс https://nova7.top для выгодных покупок! Огромный ассортимент, лучшие цены и удобные способы оплаты. Покупайте качественные товары и заказывайте с быстрой доставкой!
Louissnoda
| #
Новый формат маркетплейса https://nova4.top быстро, удобно, надежно! Лучшие предложения, современные технологии и простой интерфейс. Покупайте и продавайте легко, экономя время и деньги!
DannyDic
| #
смотреть лучшие комедии 2025 онлайн hdrezka сериалы ужасы на андроиде
mtsnovosibirsksig
| #
мтс домашний интернет новосибирск
https://globalhospitalitycareer.com/employers/i-medconsults4572/
мтс тв
affiliate programs_hgvr
| #
Premium affiliate programs in online marketing https://affiliate-c1.com/.
https://www.harvard.edu/
| #
# Harvard University: A Legacy of Excellence and Innovation
## A Brief History of Harvard University
Founded in 1636, **Harvard University** is the oldest and one of the most
prestigious higher education institutions in the United States.
Located in Cambridge, Massachusetts, Harvard has built a global reputation for
academic excellence, groundbreaking research, and influential alumni.
From its humble beginnings as a small college established to educate clergy, it has
evolved into a world-leading university that shapes the future across various disciplines.
## Harvard’s Impact on Education and Research
Harvard is synonymous with **innovation and intellectual leadership**.
The university boasts:
– **12 degree-granting schools**, including the renowned
**Harvard Business School**, **Harvard Law School**,
and **Harvard Medical School**.
– **A faculty of world-class scholars**,
many of whom are Nobel laureates, Pulitzer Prize winners, and pioneers in their fields.
– **Cutting-edge research**, with Harvard leading initiatives in artificial intelligence,
public health, climate change, and more.
Harvard’s contribution to research is immense, with billions of dollars allocated to scientific discoveries
and technological advancements each year.
## Notable Alumni: The Leaders of Today and Tomorrow
Harvard has produced some of the **most influential figures** in history,
spanning politics, business, entertainment, and science.
Among them are:
– **Barack Obama & John F. Kennedy** – Former U.S.
Presidents
– **Mark Zuckerberg & Bill Gates** – Tech visionaries (though Gates did not graduate)
– **Natalie Portman & Matt Damon** – Hollywood icons
– **Malala Yousafzai** – Nobel Prize-winning activist
The university continues to cultivate future leaders who shape industries and drive global progress.
## Harvard’s Stunning Campus and Iconic Library
Harvard’s campus is a blend of **historical charm and modern innovation**.
With over **200 buildings**, it features:
– The **Harvard Yard**, home to the iconic **John Harvard Statue** (and
the famous “three lies” legend).
– The **Widener Library**, one of the largest university libraries in the world, housing **over 20 million volumes**.
– State-of-the-art research centers, museums, and performing arts venues.
## Harvard Traditions and Student Life
Harvard offers a **rich student experience**, blending academics with vibrant traditions, including:
– **Housing system:** Students live in one of 12 residential houses,
fostering a strong sense of community.
– **Annual Primal Scream:** A unique tradition where students de-stress by running through Harvard Yard before finals!
– **The Harvard-Yale Game:** A historic football rivalry that unites alumni and students.
With over **450 student organizations**, Harvard students engage in a diverse range of extracurricular activities, from entrepreneurship to
performing arts.
## Harvard’s Global Influence
Beyond academics, Harvard drives change in **global policy, economics, and technology**.
The university’s research impacts healthcare, sustainability, and artificial intelligence,
with partnerships across industries worldwide. **Harvard’s endowment**, the largest of any university, allows it
to fund scholarships, research, and public initiatives, ensuring a legacy of
impact for generations.
## Conclusion
Harvard University is more than just a school—it’s a **symbol of
excellence, innovation, and leadership**. Its **centuries-old traditions, groundbreaking discoveries, and
transformative education** make it one of the most influential
institutions in the world. Whether through its distinguished
alumni, pioneering research, or vibrant student life, Harvard continues to shape the future in profound ways.
Would you like to join the ranks of Harvard’s legendary
scholars? The journey starts with a dream—and an application!
https://www.harvard.edu/
PrinceMep
| #
Портал с ответами https://online-otvet.site по всем школьным домашним предметам. Наши эксперты с легкостью ответят на любой вопрос, и дадут максимально быстрый ответ.
DonaldWem
| #
Руководство по настройке https://amnezia.dev VPN-сервера! Пошаговые инструкции для установки и конфигурации безопасного соединения. Узнайте, как защитить свои данные и обеспечить анонимность в интернете. Настройте VPN легко!
Gregoryfesee
| #
Сауна очищает организм https://sauna-broadway.ru выводя токсины через пот, укрепляет иммунитет благодаря перепадам температуры, снимает стресс, расслабляя мышцы и улучшая кровообращение. Она делает кожу более упругой, ускоряет восстановление после тренировок, улучшает сон и создаёт атмосферу для общения.
Earn Free Robux
| #
Good post! Do you want to know how to earn free robux? If yes, I suggested one post about earn free robux .To acquire free Robux, one can explore various methods such as participating in promotional events, engaging in online surveys, or utilizing specific gaming platforms that offer rewards. Visit rhis post to get more info.
Donaldopela
| #
Православный форум https://prihozhanka.ru для женщин! Общение о вере, молитве, семье, воспитании и роли женщины в православии. Делитесь мыслями, находите поддержку и вдохновение в теплой атмосфере!
Robertglart
| #
Korean cosmetics http://japan-lke.com/home.php?mod=space&uid=135552 perfect skin without effort! Innovative formulas, Asian traditions and visible results. Try the best skin care products right now!
MichaelAgica
| #
selector casino вход
ArthurMaigh
| #
селектор казино
Jamesjat
| #
https://www.suzuki1909.com/blog/otzyvy-o-suzuki-s-cross-nadezhnost-i-komfort-na-dorogah/
Larrynon
| #
селектор казино зеркало
Thomassef
| #
selector casino войти
teacher xxx video_mcOi
| #
student pron video https://www.teacherporntube.com .
Elektrokarniz_kcma
| #
гибкий электрокарниз привод prokarniz11.ru .
Elektrokarniz_tfMi
| #
карниз электро карниз электро .
Elektrokarniz_mnea
| #
электрические гардины электрические гардины .
Elektrokarniz_dzet
| #
электронный карниз для штор электронный карниз для штор .
Charlesjat
| #
Хочешь купить ПАВ в Москве? Нова маркетплейс – ищи в Яндекс!
mtsekaterinburgsig
| #
мтс подключение екатеринбург
https://skillnaukri.com/employer/inetrnet-domashnij-ekb-2/
мтс интернет
Felipebraby
| #
Хочешь купить ПАВ в СПБ? Нова маркетплейс – ищи в Яндекс!
RobertFaist
| #
leebet casino войти
TimothyMet
| #
leebet casino
PhilipMooxy
| #
leebet casino вход
mtsekaterinburgsig
| #
мтс подключение екатеринбург
https://empleosrapidos.com/companies/inetrnet-domashnij-ekb-2/
мтс телевидение екатеринбург
Sound Button
| #
Great article. Are you curious about choosing the most impactful sound effects? If so, Sound plays a crucial role in Roblox, shaping the atmosphere, enhancing gameplay, and creating lasting memories. With soundbuttons—a fantastic collection of sound effects—you can add the perfect audio cue to bring your Roblox world to life. Check out the blog post to discover Roblox’s most iconic sound effects.
MauroAdoca
| #
Советую приобрести прицел с дальномером, точность стрельбы стала намного выше – https://opticheskie-pricely-i-binokli.ru/
online unblocked games
| #
Good Post! Do you enjoy playing unblocked Roblox games? If so, If you’re looking for more free online unblocked game, visit GamesCentral, where you’ll find a massive selection of games across different genres. Check out the full article to learn how these platforms keep your gaming experience seamless!
the dumb test
| #
This post is amazing! Are you in a relationship and wondering about your compatibility with your partner? If so, you can take the dumb test. It’s a fun and easy way to see how well you two vibe. Playful moments like these can add excitement to your relationship. Check out the site for more information!
mtsekaterinburgsig
| #
мтс подключить
https://www.yiyanmyplus.com/companies/inetrnet-domashnij-ekb-3/
мтс подключить
zaymtulaivah
| #
Нужны деньги безотлагательно? Получите кредит мгновенно без поручителей на https://zaym-tula.ru/ без отказа! Оставьте регистрацию и получите финансы немедленно!
streetlighting
| #
outdoor lighting control systems street lighting control system
Jameslyday
| #
Сауна очищает организм https://sauna-broadway.ru выводя токсины через пот, укрепляет иммунитет благодаря перепадам температуры, снимает стресс, расслабляя мышцы и улучшая кровообращение. Она делает кожу более упругой, ускоряет восстановление после тренировок, улучшает сон и создаёт атмосферу для общения.
最佳Binance推荐代码
| #
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
Thomasles
| #
профессиональный лечебный массаж Ивантеевка расслабляющие техники для здоровья и красоты. Помогаем снять стресс, улучшить кровообращение и восстановить силы. Запишитесь прямо сейчас!
Elektrokarniz_ytet
| #
электрические гардины электрические гардины .
Michaelnat
| #
https://ourhonda.com/
Elektrokarniz_zboa
| #
карниз с электроприводом карниз с электроприводом .
Elektrokarniz_wspt
| #
карниз для штор электрический карниз для штор электрический .
mtsekaterinburgsig
| #
мтс домашний интернет
https://impactosocial.unicef.es/employer/inetrnet-domashnij-ekb-2/
сайт мтс екатеринбург
PeterPhync
| #
автоматы с бесплатным депозитом фриспины в слотах
Gerardket
| #
Женский онлайн портал https://brjunetka.ru для вдохновения! Полезные советы по стилю, уходу за собой, здоровью и семейной жизни. Будьте в курсе трендов, находите мотивацию и делитесь опытом!
Davidoraro
| #
Портал с ответами https://online-otvet.site на все школьные предметы! Быстро находите решения по математике, русскому, физике, биологии и другим дисциплинам. Готовые ответы, разборы задач и помощь в учебе для всех классов
smm-panel
| #
boost of instagram free boost of subscribers in telegram
Donalddef
| #
Элитный коттеджный поселок https://parkville-zhukovka-poselok.ru в Жуковке! Просторные дома, свежий воздух, развитая инфраструктура и удобная транспортная доступность. Создайте уютное пространство для жизни в живописном месте!
FloydEvosy
| #
stehovani trendy stehovani vyrobnich hal
mtsekaterinburgsig
| #
мтс домашний интернет екатеринбург
https://www.atlantistechnical.com/employer/inetrnet-domashnij-ekb/
провайдер мтс
Rylonnie shtori s elektroprivodom_cvSa
| #
электрические рулонные шторы купить электрические рулонные шторы купить .
Shtori s elektroprivodom_uvki
| #
шторы на электроприводе шторы на электроприводе .
smm-panel
| #
tg followers boost tt followers boost
DonaldAbumn
| #
Все о строительстве https://decor-kraski.com.ua и ремонте в одном месте! Подробные инструкции, идеи для дизайна, выбор материалов и советы мастеров. Сделайте свой дом удобным, стильным и долговечным!
HermandaT
| #
Портал про строительство https://goodday.org.ua и ремонт! Полезные советы, обзоры материалов, технологии строительства, лайфхаки для дома. Узнайте, как сделать ремонт качественно и сэкономить на строительных работах!
mtsekaterinburgsig
| #
мтс тв екатеринбург
https://thathwamasijobs.com/companies/inetrnet-domashnij-ekb-3/
мтс домашний интернет екатеринбург
jozz casino
| #
Ответственная игра: как не стать зависимым от казино
Установите лимиты на бюджет.
Прежде чем зайти в игровую среду, определите сумму денег, которую вы готовы
потратить, и строго соблюдайте этот предел.
Разделите сумму на игровые сессии,
чтобы избежать крупных потерь за
один раз.
Определите временные рамки.
Установите четкое время для ваших игровых сеансов.
Используйте таймер, чтобы напомнить себе о необходимости прекратить участие
и не останавливаться дольше, чем планировалось.
Не используйте деньги для других нужд.
Избегайте тратить средства, предназначенные для важных вещей, таких
как оплата счетов или покупка продуктов.
Игровые средства должны быть отдельными от ваших финансовых
обязательств.
Развивайте осознанность.
Обращайте внимание на свои эмоции во время игры.
Если вы начинаете чувствовать стресс
или напряжение, это признак того, что стоит остановиться.
Оцените своё состояние и не бойтесь взять паузу.
Общайтесь с окружающими.
Делитесь своим опытом с друзьями или близкими.
Обсуждение является важным аспектом, помогающим понять собственные привычки.
Поддержка со стороны может
служить дополнительным стимулом для
поддержания контроля.
Поддерживайте разнообразие интересов.
Занимайтесь хобби и активностями, которые приносят удовольствие
и не связаны с риском потерь. Это поможет сохранить
баланс и не зацикливаться на азартных развлечениях.
Методы самоконтроля для игроков в азартные заведения
Определите лимиты. Установите
четкие границы для денежных
затрат, времени, проведенного за азартными играми.
Запишите эти лимиты и придерживайтесь их, даже если возникает
соблазн продолжить.
Будьте бдительны к своему состоянию.
Analyse (анализируйте) свое эмоциональное состояние перед игрой.
Если чувствуете усталость,
стресс или грусть, отложите участие
в играх на некоторое время.
Используйте таймер. Установите таймер, который будет напоминать вам
о необходимости сделать перерыв.
Это поможет избежать длительного пребывания за игровым столом и сохранит вашу концентрацию.
Обратитесь за поддержкой. Общайтесь с друзьями или близкими об азартных увлечениях.
Открытый разговор может
помочь найти решение, если вы заметите
негативные изменения
в своем поведении.
Избегайте триггеров. Определите ситуации
или места, которые могут спровоцировать желание
участвовать в азартных играх.
Минимизируйте контакт с ними для снижения соблазна.
Занимайтесь альтернативными хобби.
Найдите увлечения, которые приносили бы вам радость и удовлетворение.
Это может быть спорт, творчество или саморазвитие, тем самым заполняя время,
которое могло бы уйти на азарт.
Поддерживайте физическую активность.
Регулярные физические нагрузки способствуют улучшению психоэмоционального состояния.
Занятия спортом помогут вам управлять стрессом и повышать умственную ясность.
Обучение и осведомленность.
Изучайте материалы о воздействии азартных игр на
личность. Понимание риска и последствий поможет принимать более обоснованные решения и избежать
негативных последствий.
Знаки проблемной игры: распознание зависимости
вовремя
Обратите внимание на изменения в финансовом поведении.
Если расходы на развлечения начинают превышать ваш бюджет, это сигнал о
возможной проблеме. Убедитесь, что у вас есть четкое представление о своих финансах
и регулярных расходах.
Следите за временем, проведенным
за активностью. Если вы не можете
отложить игру даже при необходимости выполнить важные дела
или общаться с близкими, это может указывать на нежелательное увлечение.
Обращайте внимание на эмоциональное состояние.
Частые переключения настроения,
раздражительность или эйфория связаны с игрой и могут свидетельствовать о её чрезмерной роли в жизни.
Осознание своих эмоций поможет определить,
когда наблюдаются опасные признаки.
Анализируйте ваши отношения.
Проблемы с родными, друзьями или коллегами из-за увлечения могут указывать на потенциальные последствия.
Если конфликты становятся регулярными, стоит задуматься о своих приоритетах.
Контролируйте мысли о поступках.
Если мания о выигрыше или разочаровании занимает большую часть вашего
сознания, следует обратить на это внимание и оценить влияние навязчивых идей на ваше поведение.
Отслеживайте попытки сокрытия
фактов. Если вам нужно скрывать время
занятий или свои убытки от близких, это указывает на наличие серьёзной проблемы.
Прозрачность в общении с окружающими – важный
аспект поддержки.
jozz casino
Bobbytrown
| #
Круглосуточные ритуальные услуги – помощь и поддержка в любое время https://pohoronnoe-agentstvo.kz/
rimskie shtori s elektroprivodom_qnOn
| #
автоматические римские шторы с электроприводом автоматические римские шторы с электроприводом .
elektrokarnizi somfy_omel
| #
somfy жалюзи somfy жалюзи .
SteveBaw
| #
Ремонт и строительство https://inbound.com.ua без хлопот! Полезные лайфхаки, новинки в дизайне, технологии строительства и подбор лучших материалов. Создайте комфортное жилье легко и выгодно!
Solomonbotly
| #
Ваш надежный помощник https://insurancecarhum.org в ремонте! Практичные советы, инструкции и секреты профессионалов. Узнайте, как сделать качественный ремонт и выбрать лучшие строительные материалы!
Leslieordib
| #
Портал для строителей https://hotel.kr.ua и домашних мастеров! Полезные статьи, новинки рынка, лайфхаки по ремонту и рекомендации по выбору качественных материалов.
RamonRix
| #
Инструкции по ремонту https://makprestig.in.ua подбор материалов, планирование, дизайн и советы экспертов. Станьте мастером своего дома!
Josephslatt
| #
Лучший портал по строительству https://itstore.dp.ua и ремонту! Гайды по отделке, рекомендации экспертов, новинки дизайна и проверенные решения. Все для вашего комфорта!
Jorgecoart
| #
Как сделать ремонт https://oo.zt.ua правильно? Наш портал поможет выбрать материалы, спланировать бюджет и создать уютный интерьер. Простые решения для сложных задач!
Craigsuese
| #
Профессиональные советы https://ukk.kiev.ua по ремонту и строительству! Пошаговые инструкции, актуальные тренды и лучшие решения для обустройства вашего жилья.
DanielMuh
| #
Идеи для дома https://ucmo.com.ua ремонта и строительства! Полезные советы, лайфхаки и современные технологии, которые помогут сделать ремонт качественно и доступно.
WilliamTreab
| #
Ремонт без стресса https://panorama.zt.ua Готовые решения, полезные лайфхаки, выбор материалов и идеи для дома. Делаем ремонт комфортным и доступным!
DanielMuh
| #
Идеи для дома https://ucmo.com.ua ремонта и строительства! Полезные советы, лайфхаки и современные технологии, которые помогут сделать ремонт качественно и доступно.
JamesTOW
| #
Делаем ремонт правильно https://zarechany.zt.ua Разбираем все этапы – от выбора материалов до дизайна интерьера. Подробные инструкции и лайфхаки от специалистов!
StephenTon
| #
Автомобильный портал https://nerjalivingspace.com для автолюбителей! Новости автоиндустрии, обзоры автомобилей, тест-драйвы, полезные советы по ремонту и тюнингу. Будьте в курсе последних трендов и находите ответы на все авто-вопросы!
mtsekaterinburgsig
| #
мтс подключить екатеринбург
https://www.punajuaj.com/companies/inetrnet-domashnij-ekb-3/
мтс тв екатеринбург
"https://eve5.wiki/index.php/Maxstresser_77j"
| #
Hi, Neat post. There’s an issue along with your web site in web explorer, might check this?
IE still is the market leader and a large element of folks will leave out your excellent writing due to this problem.
“https://dynastyascend.com/wiki/Maxstresser_89z”
"https://westhamwiki.com/index.php/Maxstresser_89N"
| #
Does your website have a contact page? I’m having trouble locating
it but, I’d like to send you an email. I’ve got
some recommendations for your blog you might be interested
in hearing. Either way, great blog and I look forward to seeing it grow over time.
“https://adscenter.site/user/profile/GeorgiaMuy”
Kennethroste
| #
https://169.ru/mezhkomnatnye-dveri/bravo/
1win_wber
| #
1win ставки официальный сайт https://www.1win3.com.kg .
AndrewJatte
| #
Все для женщин https://elegance.kyiv.ua в одном месте! Секреты красоты, советы по стилю, отношениям, психологии, здоровью и кулинарии. Будьте вдохновленными и уверенными каждый день!
Williamlor
| #
Женский портал https://beautyadvice.kyiv.ua для современной женщины! Мода, красота, здоровье, отношения, кулинария и карьера. Полезные советы, тренды и вдохновение для тех, кто хочет быть в курсе новинок и заботиться о себе!
ShaneGob
| #
Онлайн мир женщины https://gracefullady.kyiv.ua красота, здоровье, успех! Полезные лайфхаки, тренды моды, секреты счастья и гармонии. Портал для тех, кто хочет быть лучшей версией себя!
Robertvoisa
| #
Портал для современной https://femaleguide.kyiv.ua женщины! Открой для себя новые идеи в моде, красоте, отношениях и саморазвитии. Полезные статьи и советы для комфортной и счастливой жизни
HarrySnich
| #
Мир женщины https://fashionadvice.kyiv.ua красота, здоровье, успех! Полезные лайфхаки, тренды моды, секреты счастья и гармонии. Портал для тех, кто хочет быть лучшей версией себя!
megafonekbWAH
| #
мегафон интернет
https://ekb-domasnij-internet-2.ru
мегафон подключение екатеринбург
Howardtrese
| #
Твой гид в мире https://happywoman.kyiv.ua женственности и гармонии! Узнай больше о моде, косметике, фитнесе, отношениях и мотивации. Все, что нужно для яркой и счастливой жизни!
1win_wcKr
| #
1 win официальный сайт 1win4.com.kg .
1win_zhsn
| #
1win официальный http://1win5.com.kg/ .
megafonekbWAH
| #
мегафон тв екатеринбург
https://ekb-domasnij-internet-3.ru
провайдер мегафон
1win_xppa
| #
1win регистрация 1win6.com.kg .
mostbet kirgizstan_pnOi
| #
mostbet online http://www.mostbet1.com.kg/ .
PatrickheW
| #
Безопасный доступ к сайту https://bsme-at.at без ограничений. Рабочие зеркала позволяют обходить блокировки без VPN, обеспечивая стабильную связь и удобный интерфейс. Следите за обновлениями, чтобы всегда оставаться в сети.
Brunoneelm
| #
Актуальные зеркала BlackSprut https://kra26.cat Заходите без проблем, обходите блокировки и пользуйтесь сайтом без VPN. Мы следим за обновлениями и всегда предоставляем свежие ссылки.
Haroldhal
| #
Всегда актуальные ссылки https://bs2bet.at для входа. Обходите блокировки легко и быстро, используя надёжные зеркала. Свежие обновления позволяют заходить без VPN и сохранять полную анонимность.
HowardDrync
| #
Проблемы с входом https://bs2best.or.at Найдите актуальные зеркала и заходите без ограничений. Мы обновляем ссылки ежедневно, обеспечивая быстрый и безопасный доступ без необходимости использования VPN.
Williamexalp
| #
BlackSprut это инновационный https://bs2best.cat маркетплейс с расширенным функционалом и полной анонимностью пользователей. Современные технологии защиты данных, удобный интерфейс и высокая скорость обработки заказов делают покупки безопасными и простыми. Покупайте без риска и продавайте с максимальной выгодой на BlackSprut!
Howarddef
| #
BlackSprut крупнейший маркетплейс https://bb2best.at где можно найти всё, что вам нужно. Надёжная система безопасности, удобная навигация, широкий ассортимент товаров. Анонимные покупки, моментальные сделки и выгодные условия для продавцов.
KennethMoive
| #
BlackSprut передовой маркетплейс https://m-bsme.at с высокими стандартами безопасности и удобной системой поиска. Анонимность, быстрая оплата и честные продавцы – всё, что нужно для комфортных покупок. Мы гарантируем защиту ваших данных и качественное обслуживание. Присоединяйтесь к BlackSprut и открывайте новые возможности!
"https://yogaasanas.science/wiki/Jurnal_69T_random"
| #
I really like what you guys are up too. Such clever work and reporting!
Keep up the excellent works guys I’ve incorporated you guys to blogroll.
“https://systemcheck-wiki.de/index.php?title=Benutzer:CliffFfa80”
KennethGloto
| #
проверенный маркетплейс https://bsme.cat предлагающий товары на любой вкус. Простая регистрация, быстрая оплата и защита сделок. Убедитесь в качестве сервиса сами!
Michaeldeava
| #
Blacksprut – современная площадка https://bs-bsme.at для безопасных покупок. Большой выбор категорий, продуманная система рейтингов и отзывов. Заходите и находите нужное легко!
AnthonyUsero
| #
Blacksprut – онлайн-маркетплейс https://bsmc.at с лучшими предложениями. Надёжные продавцы, защищённые сделки, удобный поиск. Оцените удобство покупок уже сегодня!
Timothylaf
| #
Ищете актуальные бонусы без отыгрыша? Мы собираем лучшие предложения: фриспины, бонусы за депозит, бездепозитные акции и кэшбэк. Получите максимум выгоды от игры!
megafonekbWAH
| #
провайдер мегафон
https://ekb-domasnij-internet.ru
мегафон подключение екатеринбург
JamesIcodo
| #
Replica Uhren https://www.imailen.com in Deutschland schnell und sicher per DHL Nachnahme bestellen. Nur geprufte Qualitat in EU hergestellt.
RichardEnamn
| #
Журнал об автомобилях https://setbook.com.ua всё для автолюбителей! Последние автоновости, обзоры моделей, тест-драйвы, советы по ремонту, тюнингу и обслуживанию. Узнайте всё о мире автомобилей!
Raymondexomy
| #
Главный авто-журнал https://myauto.kyiv.ua для водителей! Новости, обзоры, сравнения, тюнинг, автоспорт и технологии. Будьте в курсе последних трендов автомобильного мира!
poeshop
| #
купить сферу хаоса path of exile 2 купить дивы пое 2
ThomasLiale
| #
Все об автомобилях https://allauto.kyiv.ua в одном журнале! Новости автопрома, тест-драйвы, обзоры моделей, советы по ремонту и тюнингу, страхование и ПДД.
HectorAmibe
| #
Журнал для автолюбителей https://myauto.kyiv.ua и профессионалов! Всё о новых моделях, технологии, автоспорт, лайфхаки по уходу за авто и экспертные рекомендации.
DustinLaf
| #
Журнал об авто https://auto-club.pl.ua для тех, кто за рулем! Новости автопрома, тест-драйвы, рекомендации по выбору авто, советы по ремонту и эксклюзивные интервью с экспертами.
beelinekrasnodarTet
| #
билайн домашний интернет
https://infodomashnij-internet-krasnodar-2.ru
билайн домашний интернет
Mostbet_hxMi
| #
мостбет ставки мостбет ставки .
Mostbet_nvEa
| #
vjcn,tn vjcn,tn .
Mostbet_rrSl
| #
букмекерская контора мостбет букмекерская контора мостбет .
beelinekrasnodarTet
| #
билайн подключить краснодар
https://infodomashnij-internet-krasnodar-3.ru
билайн подключить
VanceCam
| #
http://registr-a.ru
StefanGeX
| #
Все о машинах https://autoinfo.kyiv.ua в одном журнале! Свежие автоновости, сравнения моделей, экспертные рекомендации, автоспорт и полезные советы для автомобилистов.
Dwayneamips
| #
Журнал для автолюбителей https://avtoshans.in.ua и профессионалов! Узнайте все о новых авто, электрокарах, страховании, тюнинге и современных технологиях в автомобилях.
Darrencox
| #
Автомобильный мир https://diesel.kyiv.ua в одном журнале! Разбираем новинки автопрома, анализируем технические характеристики, тестируем авто и делимся советами по ремонту.
mostbet kirgizstan_aiEn
| #
мостбет казино играть mostbet2.com.kg .
MarcusClita
| #
Современный автомобильный https://k-moto.com.ua журнал! Читайте о трендах в автоиндустрии, новейших моделях, электромобилях, тюнинге и умных технологиях.
DouglasReema
| #
Автомобильный журнал https://orion-auto.com.ua только важные новости! Все о популярных марках, топовые авто 2024 года, электрокары, автономное вождение и тенденции рынка.
TimothyLiexy
| #
Журнал о машинах https://reuth911.com для настоящих ценителей авто! Обзоры, рейтинги, полезные статьи о ремонте, тюнинге и современных автомобильных технологиях.
KennethCip
| #
Автомобильный мир https://prestige-avto.com.ua в одном журнале! Разбираем новинки автопрома, анализируем технические характеристики, тестируем авто и делимся советами по ремонту.
beelinekrasnodarTet
| #
сайт билайн краснодар
https://infodomashnij-internet-krasnodar.ru
билайн цены
spider hoodie_tyel
| #
sp5der hoodie red https://spiderhoodie-us.com/spider-hoodies/ .
GordonApask
| #
Твой авто-гид https://troeshka.com.ua в мире машин! Обзоры новых моделей, тест-драйвы, советы по ремонту и тюнингу, автоновости и технологии будущего. Все, что нужно автолюбителю!
RaymonAgors
| #
Все об автомобилях https://tvk-avto.com.ua на одном портале! Новинки мирового автопрома, тест-драйвы, автострахование, электрокары и полезные статьи для каждого водителя.
BobbyLyhon
| #
Журнал про автолюбители https://tuning-kh.com.ua новости, тесты, обзоры! Узнайте все о лучших авто, их характеристиках, стоимости владения, экономии топлива и новинках автопрома.
StephenFrade
| #
Твой идеальный женский https://family-site.com.ua журнал! Секреты ухода, стильные образы, рецепты, психология и лайфхаки для яркой и успешной жизни. Вдохновение каждый день!
Dennysnubs
| #
Актуальные новости https://www.moscow.regnews.info Московского региона! Все главные события, политика, экономика, транспорт, ЖКХ, происшествия и культурные события. Будьте в курсе того, что происходит в вашем городе!
EddiePaL
| #
Главный женский журнал https://amideya.com.ua о стиле и успехе! Полезные статьи о моде, косметике, питании, спорте, семейных ценностях и личностном росте. Читай и развивайся вместе с нами!
Davidsom
| #
Твой идеальный женский https://femalesecret.kyiv.ua журнал! Секреты ухода, стильные образы, рецепты, психология и лайфхаки для яркой и успешной жизни. Вдохновение каждый день!
MichaelKap
| #
Портал для женщин https://feminine.kyiv.ua твой путеводитель в мире стиля и успеха! Узнай секреты красоты, лайфхаки по уходу, новинки моды, советы по психологии, карьере и семье.
Billyawali
| #
Твой женский портал https://girl.kyiv.ua о стиле и гармонии! Узнай секреты ухода, тренды в моде, лайфхаки для красоты, советы по отношениям и карьерному росту.
whore
| #
детское гей порно магазин купить меф
Waltersow
| #
Главный женский портал https://horoscope-web.com будь в центре трендов! Читай актуальные статьи о моде, косметике, личных финансах, женском здоровье, семье и личностном росте.
DanielMus
| #
Портал для женщин https://lolitaquieretemucho.com которые ценят себя! Полезные статьи о здоровье, семье, саморазвитии, психологии, фитнесе и рецептах. Будь уверенной, счастливой и успешной!
вован казино скачать
| #
Выигрыши выводятся моментально, проверил на личном опыте.
вован казино скачать
Stihi dlya detei_dcma
| #
Пословицы и поговорки для детей Пословицы и поговорки для детей .
Zamena tyrbini razvod_lgSn
| #
Как заменить турбину на машине и не переплатить втрое больше. Типичная схема развода на замену турбины. Будьте осторожны. Подробности тут Как заменить турбину на машине и не переплатить втрое больше. Типичная схема развода на замену турбины. Будьте осторожны. Подробности тут .
SOYT_rfkt
| #
заявка на соут http://www.sout095.ru/ .
beelinekrasnodarTet
| #
билайн интернет краснодар
https://plus-domashnij-internet-krasnodar-2.ru
сайт билайн краснодар
JimmyFex
| #
Портал для женщин https://nicegirl.kyiv.ua которые любят себя! Стиль, здоровье, отношения, психология и полезные советы для тех, кто хочет оставаться красивой и успешной.
Ernesthax
| #
Твой путеводитель https://mirlady.kyiv.ua в мире женских секретов! Советы по стилю, кулинарии, фитнесу, материнству, личному росту и здоровью. Всё, что нужно для счастливой жизни!
Willielinue
| #
Современный женский портал https://presslook.com.ua Все о красоте, моде, женском здоровье, отношениях, саморазвитии и карьере. Читай, вдохновляйся и меняй свою жизнь к лучшему!
Jameshut
| #
Твой идеальный https://prettywoman.kyiv.ua женский портал! Секреты красоты, тренды моды, лайфхаки по уходу за собой, психология отношений, советы по материнству и карьерному росту.
zaymbezproverokvah
| #
Нужны капитал срочно? Получите онлайн-заем на пластиковую карту за момент. https://zaym-bez-proverok.ru/ Оформите форму без документов и получите положительный ответ уже сегодня!
JimmyCance
| #
Сайт для женщин https://ramledlightings.com будь лучшей версией себя! Читай о моде, красоте, психологии, отношениях, материнстве и женском здоровье. Найди вдохновение и полезные советы для каждого дня!
JamesCalia
| #
Лучший портал для родителей https://geog.org.ua и детей! Читайте о воспитании, обучении, здоровье, детской психологии, играх и семейном досуге. Советы экспертов и практические рекомендации.
Manuelagido
| #
Портал о детях https://mch.com.ua полезно для родителей! Воспитание, здоровье, развитие, обучение, досуг, игры и семейные традиции. Экспертные советы, лайфхаки и полезные материалы для гармоничного роста малыша.
Arnoldgrews
| #
Твой женский сайт https://musicbit.com.ua о стиле, здоровье и вдохновении! Узнай секреты красоты, следи за модными новинками, развивайся, читай о психологии отношений и оставайся в гармонии с собой.
beelinekrasnodarTet
| #
билайн интернет
https://plus-domashnij-internet-krasnodar.ru
билайн подключить краснодар
zaymbezproverokvah
| #
Нужны деньги срочно? Получите микрозайм на счет в банке за короткий срок. https://zaym-bez-proverok.ru/ Оформите анкету без документов и получите положительный ответ уже сегодня!
Robertben
| #
Справочник лекарственных https://mikstur.com средств – полная информация о медикаментах! Описания, показания, противопоказания, дозировки, аналоги и инструкции по применению.
DuaneMiz
| #
Портал о здоровье https://fines.com.ua все, что нужно для крепкого организма! Советы врачей, профилактика болезней, здоровое питание, спорт, психология, народная медицина и лайфхаки для долгой и активной жизни.
turkof
| #
турецкие сериалы на русском языке турецкие сериалы смотреть
Jeffreyelolo
| #
сериалы на турецком языке онлайн смотреть турецкие сериалы онлайн
Kennethdob
| #
турецкие сериалы бесплатно турецкие сериалы смотреть
Scottfouch
| #
смотреть лучшие турецкие сериалы смотреть онлайн турецкие сериалы
LloydKic
| #
турецкие сериалы бесплатно смотреть турецкие сериалы онлайн бесплатно
beelinekrasnodarTet
| #
билайн домашний интернет краснодар
https://plus-domashnij-internet-krasnodar-3.ru
провайдер билайн
ShaneRub
| #
турецкие сериалы онлайн турецкий сериал смотреть
Clydeglalo
| #
турецкие сериалы на турецком языке смотреть турецкие сериалы онлайн бесплатно
ToneyWax
| #
сериалы на турецком языке онлайн смотреть турецкий сериал
Samuelbig
| #
турецкие сериалы бесплатно турецкие сериалы онлайн
Пансионат «Уют» Киев Вышгород
| #
Будинок престарілих Пансіонат «Затишок» Вишгород
https://dom-prestarelyh.kiev.ua/ru/
⏩ Догляд, оздоровлення, реабілітація літніх людей. Догляд за лежачими хворими ⏩ Так само, ми надаємо послуги медичного транспортування з України і за кордоном, пишіть нам на електронну пошту або дзвоніть
Перевезення Хворих Київ, Вишгород, Україна
Дом престарелых Пансионат «Уют» Вышгород ⏩ Специализируется по уходу, оздоровлению и реабилитацией за пожилыми людьми и за лежачими больными. ⏩ Так же, мы предоставляем услуги медицинской транспортировки по Украине и за границей, пишите нам на электронную почту или звоните.
#ПансионатКиев
#ДомПрестарелыхВышгород
#ПансіонатВишгород
#БудинокПохилогоВікуВишгород
Перевезення хворих Львів
| #
Перевезення хворих за кордон
https://a-med.com.ua/services/mizhnarodni-perevezennya/
Перевезення хворих Львів • Приватна платна швидка медична допомога Львів ⚕️ Транспортування лежачих, сидячих та важких хворих Львів – Україна ☎️ +380680177630 ♥ Кваліфіковане медичне транспортування ➡ Медичний супровід хворих Львів ⏩ Міжнародне перевезення хворих ⏩ Перевезення закордон ➡ Львів ➡ А-Мед • Transportation of patients abroad
Транспортування лежачих, сидячих та тяжких хворих Львів – Україна ☑️ Міжнародне перевезення хворих ⏩ Приватний медичний транспорт
#МедичнийТранспортКиїв
#МедичнийТранспортЛьвів
#МедичнийТранспортХарків
#Транспортное
#МедичнийТранспортДніпро
#ПеревезенняХворихЛьвів
#ПеревезенняТяжкихХворихЛьвів
#ПеревезенняХворихЗакордон
Перевезення хворих за кордон
Натяжные потолки Одесса.
| #
Натяжные потолки Одесса и Одесская область.
https://www.tecko.in.ua/
✓ Ремонт, установка натяжных потолков.
✓ Натяжные потолки любых видов индивидуально и под заказ.
✓ Натяжные стены в Одессе.
✓ Подвесные потолки в Одессе
Натяжні стелі Одеса – TEKO ⏩ Актуальні поради з натяжних стель ⭐ Натяжні стелі за типом покриття ☑️ Вибираємо натяжні стелі з малюнком ➤ Види барвників для натяжних стель ♥ найпопулярніші теми натяжних стель
#НатяжныеПотолкиОдесса
#НатяжныеПотолкиОдессаЦена
#ПодвесныеПотолкиОдесса
#НатяжныеСтеныОдесса
#НатяжныеПотолкиТЕКО
#НатяжныеПотолкивОдессе
#НатяжныеПотолкиСделайСам
#НатяжныеПотолки
#ПотолкиОдесса
#НатяжныеПотолкиОдессаЦена
#КупитьНатяжныеПотолкиОдесса
Натяжные потолки Одесса – TEKO
Перевезення хворих Львів Мед 103
| #
Перевезення хворих Львів Мед103
https://med103.com/cities/lviv/
♦ Приватна платна швидка медична допомога Львів ⚕️ Транспортування лежачих, сидячих та важких хворих Україна ☎️ +380738870303 ♥ Кваліфіковане медичне транспортування ➡ Медичний супровід хворих ⏩ Міжнародне перевезення хворих ⏩ Львів ➡ Львів ➡ Мед 10
Транспортування лежачих, сидячих та тяжких хворих Львів – Україна ☑️ Міжнародне перевезення хворих ⏩ Приватний медичний транспорт
#МедичнийТранспортКиїв
#МедичнийТранспортЛьвів
#МедичнийТранспортХарків
#МедичнийТранспортОдеса
#МедичнийТранспортДніпро
#ПеревезенняХворихУкраїна
Частная платная скорая медицинская помощь Днепр ⚕️ Транспортировка лежачих, сидячих и тяжелых больных Украина ☎️ +380738870303 ♥ Квалифицированная медицинская транспортировка ➡ Медицинское сопровождение больных ⏩ Международная перевозка больных ⏩ Частный медицинский транспорт ♥ Med103 ➡ Киев ➡ Одесса ➡ Львов • Днепр • Харьков Украина
Транспортировка лежачих, сидячих и тяжелых больных Украина ☑️ Международная перевозка больных ⏩ Частный медицинский транспорт
#МедицинскийТранспортКиев
#МедицинскийТранспортЛьвов
#МедицинскийТранспортХарьков
#МедицинскийТранспортОдесса
#МедицинскийТранспортДнепр
#ПеревозкаБольныхУкраина
Private paid ambulance ⚕️ Transportation of bedridden, sitting and seriously ill patients Ukraine ☎️ +380738870303 ♥ Qualified medical transportation ➡ Medical support for patients ⏩ International transportation of patients ⏩ Private medical transport ♥ Med103 ➡ Kyiv ➡ Odessa ➡ Lviv • Dnepr • Kharkov Ukraine
Міжнародне медичне перевезення. Країни
Німеччина
Румунія
Італія
Польща
Молдова
Пансионат «Уют» Днепр
| #
Дом престарелых Пансионат «Уют» Днепр
https://uyt.dp.ua/ru/
Будинок престарілих Пансіонат «Затишок» Дніпро ⏩ Спеціалізується по догляду, оздоровленню і реабілітацією за літніми людьми і за лежачими хворими ⏩ Так само, ми надаємо послуги медичного транспортування з України і за кордоном, пишіть нам на електронну пошту або дзвоніть
⏩ Специализируется по уходу, оздоровлению и реабилитацией за пожилыми людьми и за лежачими больными. ⏩ Так же, мы предоставляем услуги медицинской транспортировки по Украине и за границей, пишите нам на электронную почту или звоните.
#ПансионатДнепр
#ДомПрестарелыхДнепр
#ПансіонатДніпро
#БудинокПохилогоВікуДніпро
BrianMop
| #
серия сезон турецкий сериал https://proturkish.biz
MichaelPes
| #
турецкий сериал все серии турецкие сериалы смотретя
beelinekrasnodarTet
| #
билайн телевидение краснодар
https://plus-domashnij-internet-krasnodar-2.ru
билайн тарифы краснодар
Rylonnie shtori s elektroprivodom_rzpl
| #
рулонные шторы автоматические купить рулонные шторы автоматические купить .
mostbet kirgizstan_cqKt
| #
мостбет вход регистрация https://www.mostbet3.com.kg .
mostbet kirgizstan_woSl
| #
mostbet.com skachat mostbet4.com.kg .
obychenie po ohrane tryda moskva_lspa
| #
дистанционное обучение работника по охране труда Москва дистанционное обучение работника по охране труда Москва .
SOYT_zfsl
| #
соут специальная оценка условий соут специальная оценка условий .
JosephTor
| #
топ лучших казино
IsraelCow
| #
лицензионные слоты игровые автоматы рейтинг лучших сайтов россии
Michaelgit
| #
online casino eldorado casino
beelinekrasnodarTet
| #
билайн подключить
https://plus-domashnij-internet-krasnodar.ru
билайн домашний интернет краснодар
Roberttraro
| #
Мы подобрали часто задаваемые вопросы пользователей из Казахстана на тему https://promokod-na-1xbet.com/ и их решения. Ввод промокодов при создании аккаунта и начальном депозите обеспечивает возможность значительно увеличить баланс и получить больше возможностей для игры.
네이버 아이디 구매
| #
https://consolebang.com/members/%EB%84%A4%EC%9D%B4%EB%B2%84-%EC%95%84%EC%9D%B4%EB%94%94-%EA%B5%AC%EB%A7%A4.34257/#about
비아그라 구매
| #
https://xn--w5-o02ik82a9kav54aokmxvc.mystrikingly.com/blog/3f69fae99e6
beelinekrasnodarTet
| #
билайн подключение
https://plus-domashnij-internet-krasnodar-3.ru
билайн интернет краснодар
накрутка подписчиков Инстаграм без заданий бесплатно
| #
Продвижение в Instagram стало проще с накруткой!
накрутка подписчиков Инстаграм без заданий бесплатно
Waltertop
| #
https://aldk24.ru/
накрутка зрителей на стрим Твич
| #
Можно ли накручивать зрителей постепенно?
накрутка зрителей на стрим Твич
JamesNeign
| #
New Retro Casino
JamesTog
| #
kinogo новинки kinogo фильмы для компьютера
RobertKiz
| #
kinogo боевики 2025 киного фильмы для мобильных устройств
Ronaldvek
| #
Booi Casino
Kevinfipse
| #
казино с пополнением с криптой
accurateautobodyrepairlmailm
| #
Our vehicle tuning services are designed to enhance your performance. We offer unique upgrades that improve the efficiency and aesthetic of your auto. Whether you’re interested in suspension modifications or tuning specific parts, we provide top solutions for every need. Trust our experts to deliver reliable results that will revive your ride. For more details, visit our website at https://accurateautobodyrepair.com/ and discover how we can help you.
Nakrytka podpischikov TG_jcPl
| #
накрутка подписчиков ТГ накрутка подписчиков ТГ .
Nakrytka Tvich_ukMr
| #
программа для накрутки зрителей Твич программа для накрутки зрителей Твич .
1win md_qlea
| #
1win moldova download 1win moldova download .
1win md_koKr
| #
?nregistrare 1win https://1win3.md .
Merlezew
| #
акции вб
MichaelDycle
| #
Discover endless entertainment at Fmovies! Stream the latest movies, TV shows, and series in HD, all for free. Enjoy a user-friendly interface, fast streaming, and a vast library updated daily. Your favorite content is just a click away https://2fmoviesto.com/
beelinekrasnodarTet
| #
билайн тв краснодар
https://plus-domashnij-internet-krasnodar-2.ru
провайдер билайн
Kenneth_Greno
| #
накрутка подписчиков Ютуб без отписок
accurateautobodyrepairlmailm
| #
Our motor tuning services are designed to enhance your performance. We offer personalized upgrades that improve the engine and design of your auto. Whether you’re interested in engine modifications or upgrading specific parts, we provide high solutions for every need. Trust our experts to deliver flawless results that will boost your ride. For more details, visit our website at https://accurateautobodyrepair.com/ and discover how we can help you.
create binance account
| #
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
RobertJEM
| #
ключ активации виндовс 11
Melvingor
| #
Непродовольственные товары Услуги перевозки в интернет-магазине товаров, не относящихся к продуктовому сегменту.
Edwardmak
| #
Автопортал https://avtogid.in.ua Автогiд сайт с полезными советами для автовладельцев. Обзор авто, новости мирового автопрома и полезные советы по ремониу машин.
네이버 아이디 구매
| #
https://naveridbuy.exblog.jp/35878128/
LarryBex
| #
Вавада казино
1vin_pwsl
| #
казино lucky jet http://1win1.com.kg/ .
DustinZen
| #
https://deseko.ru/
Леон казино
| #
Реальные отзывы об онлайн казино: где
искать?
Чтобы составить объективное представление о различных игровых заведениях в интернете,
стоит обратить внимание на специализированные форумы и сайты.
Эти площадки, как правило, собирают мнения настоящих пользователей, которые делятся
своим опытом и рассказывают о том, что на самом
деле происходит за кулисами.
Ключевые ресурсы, такие как Gamblers’ Review или CasinoGuru, предоставляют качественную информацию и
ранжируют заведения по различным критериям.
Также рекомендую воспользоваться социальными сетями,
такими как Facebook и Reddit. Там часто можно найти живое обсуждение
платформ, а также задать вопросы опытным игрокам.
Обратите внимание на группы, посвященные играм на деньги,
где участники делятся как
положительными, так и отрицательными моментами.
Пароли к успеху – это тщательный анализ найденной
информации и скептический подход к ней.
Не забывайте проверять дату публикации мнений, так как рынок азартных игр
постоянно меняется, и информация, актуальная в прошлом, может быть устаревшей.
Доступ к свежим данным поможет избежать неприятных сюрпризов при выборе места для игры.
Поиск проверенных платформ для
отзывов об азартных учреждениях
Ищите специализированные сайты,
которые тщательно анализируют отзывы
пользователей. Обратите внимание на
ресурсы, предлагающие независимый контент и рейтинги.
Платформы, такие как AskGamblers, CasinoGuru и Trustpilot,
содержат информацию о различных заведениях, включая
комментарии клиентов и оценки.
Проверьте наличие форумов и сообществ, где игроманы обсуждают свой опыт.
Сайты, такие как Reddit, содержат разделы,
посвященные игорным заведениям, где пользователи делятся
конкретными случаями.
Изучите наличие блогов и YouTube-каналов, где эксперты делают обзоры и предлагают детализированные
мнения о работе платформ.
Опытные игроки иногда публикуют свои впечатления о
конкретных игорных площадках, что может быть полезным для
принятия решения.
Обратите внимание на профессиональные игровые порталы, которые проводят тщательную проверку заведений перед публикацией информации о них.
Убедитесь, что платформа имеет документированные исследования и
сравнительные анализы.
Фокусируйтесь на отзывах, которые отмечают
конкретные аспекты работы заведения, такие как скорость выплат,
качество службы поддержки и разнообразие игрового контента.
Это поможет получить более полное
представление о репутации заведения.
Анализ отзывов: как отличить
правду от лжи
Для оценки подлинности комментариев, проверьте источники.
Начните с площадок, имеющих
репутацию, таких как форумы и специализированные сайты,
где пользователи обсуждают свои впечатления.
Сравните несколько откликов.
Если в большинстве случаев звучат схожие проблемы или положительные моменты,
это повышает вероятность статистической достоверности.
Однако уклончивые сообщения с общими фразами могут указать на намерение скрыть истинный опыт.
Отслеживайте даты публикаций.
Свежие упоминания могут быть полезнее, так как они отражают
актуальную действительность сервиса.
Устаревшие данные могут не представлять реальной
картины.
Изучайте также поведение
авторов. Если пользователь оставляет комментарии исключительно положительного или отрицательного
характера, это может говорить о предвзятости, иногда связанной с работой представителя заведения.
Ищите сбалансированные мнения.
Обратите внимание на соотношение отзывов на разных ресурсах.
Если на одном сайте преобладают негативные оценки, а на других –
исключительно положительные, это может указывать на манипуляции
с рейтингами.
Не верьте слепо. Правдивый опыт быстро становится вирусным,
в то время как купленные
мнения могут выглядеть шаблонно.
Изучая среднюю точку зрения,
выбирайте наиболее обоснованные и правдоподобные
мнения пользователей.
Леон казино
beelinekrasnodarTet
| #
провайдер билайн
https://plus-domashnij-internet-krasnodar.ru
билайн подключить краснодар
네이버 아이디 구매
| #
https://energetic-goat-dc4vlq.mystrikingly.com/blog/1b973c46327
Пинко казино
| #
Какие игры в казино самые прибыльные?
Рекомендуется обратить внимание на блэкджек, так как
он предлагает один из лучших коэффициентов для игроков.
С оптимальной стратегией, возврат к игроку (RTP) может достигать 99.5%.
Также, различные вариации, такие как
European Blackjack, могут обеспечить
ещё более высокие выигрыши за счёт меньшего
количества колод.
Покер, в частности техасский холдем, представляет собой отличную возможность для получения прибыли.
Здесь всё зависит от умений игрока.
Профессионалы могут получить значительные выигрыши, так как казино
зарабатывает на сборах, а не на проигрыше клиентов.
Автоматы могут быть прибыльными, но важно выбирать слоты с высоким RTP, часто превышающим 96%.
Например, такие модели, как Blood Suckers или Mega Joker, известны своими
хорошими выплатами, что делает их предпочтительными для многих азартных посетителей.
Рулетка также заслуживает внимания.
Игроки могут использовать стратегию,
например, ставя на наружные ставки, что обеспечивает более высокий шанс на выигрыш, хотя и с меньшими
выплатами. Европейская рулетка с одним зеро считается наиболее выгодной для участников.
Анализ возврата игроку (RTP) в различных играх казино
Различные автоматы обеспечивают разные коэффициенты RTP.
Обычно они варьируются от 85% до 98%.
Слоты с высоким RTP, как правило, более выгодны.
Например, такие как “Blood Suckers” и “Mega Joker” достигают RTP 98% и выше.
В настольных вариантах, таких как блэкджек и
рулетка, наблюдается различие в показателях.
Блэкджек, при оптимальной стратегии, может иметь RTP до 99.5%, что
делает его одним из самых заложенных механик.
В рулетке классические варианты
предлагают RTP около 97.3% для европейской версии, что превосходит американскую с её RTP
около 94.74%.
Видеопокер также показывает параметры, которые варьируются.
Например, игра “Jacks or Better” с правильной стратегией
приближается к 99.5% RTP. Важно отмечать, что чем больше знаний в стратегии,
тем выше шансы на получение прибыльных результатов.
Тематические автоматы с прогрессивным
джекпотом могут иметь низкий RTP, иногда ниже 90%.
Однако потенциальные выигрыши могут быть значительными,
что привлекает игроков, готовых рискнуть ради больших призов.
Итак, тщательно изучая RTP разных вариантов,
можно значительно улучшить шансы на успех.
Выбор автоматов или настольных игр с более высоким показателем RTP
может стать решающим фактором при
принятии решения о том, как разместить свои ставки и вывести
максимальную выгоду из игрового опыта.
Стратегии и советы для игр с высоким
шансом на выигрыш
Используйте стратегию счет карточек для блэкджека.
Это позволит увеличить вероятность выигрыша, отслеживая соотношение высоких
и низких карт в колоде. При этом важно сохранять спокойствие и придерживаться своей тактики.
В рулетке выбирайте ставки на цвет или
четность вместо отдельных
чисел. Такой подход значительно снижает
риск потерь и увеличивает шансы на
победу.
Во время игры в покер воспользуйтесь позиционной стратегией.
Игроки на поздней позиции получают преимущество, так как имеют возможность оценить действия своих оппонентов перед принятием
решения.
Обязательно устанавливайте лимиты
для проигрышей и выигрышей.
Это поможет контролировать бюджет и избежать крупных потерь.
Понимание коэффициентов и выплат в слотовых машинах повысит
шансы на успех. Ищите модели с высоким процентом возврата игроку (RTP) и
низкой дисперсией.
Знайте и используйте бонусы и акции,
которые предлагают заведения.
Они могут значительно увеличить игровую линию
и обеспечить дополнительные возможности для выигрыша.
Обращайте внимание на правила конкретного формата,
чтобы избегать ошибок, которые могут стоить вам денег.
Понимание механики поможет принимать более обоснованные решения.
Регулярно практикуйтесь в бесплатных версиях, чтобы улучшить
свои навыки и выявить стратегии,
которые работают для вас
наилучшим образом. Это даст возможность уверенно подходить к игре на деньги.
Пинко казино
nakrutk_Spupt
| #
накрутка лайков в ВК бесплатно
Пинко казино
| #
Лучшие онлайн казино 2025 года
Рекомендуем обратить внимание на несколько выдающихся платформ в 2025 году, которые предлагают привлекательные условия
и высококачественный сервис. В первую
очередь стоит выделить Royal Slot.
Это заведение зарекомендовало себя
благодаря высокому уровню безопасности и множеству выборов
развлечений, включая новые слоты с уникальными механиками.
Еще одной примечательной площадкой является PlayZone, предлагающая
щедрые бонусы и быструю выплату выигрышей.
Бонусы на первый депозит могут достигать 200%, что является отличным мотивом для новых пользователей.
В дополнение к стандартным играм, здесь представлено множество
эксклюзивных турниров и акций.
Не стоит забывать и о WinMasters, устойчиво набирающем
популярность благодаря интуитивно
понятному интерфейсу и широкому выбору платежных методов.
Благодаря множеству отзывов
пользователей, платформа
выделяется среди конкурентов
комфортом и надежностью, что немаловажно для игроков.
Каждое заведение решает задачи своих клиентов по-разному,
поэтому рекомендуется ознакомиться с условиями при
регистрации и выбрать наиболее
подходящий для себя вариант. Поскольку ставки
сегодня стали доступнее, важно учитывать рейтинги и
отзывы, чтобы принимать взвешенные решения при выборе площадки.
Критерии выбора надежного виртуального заведения в 2025 году
Первое, на что следует обратить внимание, – наличие лицензии.
Проверяйте, выдана ли лицензия авторитетным регулятором, таким как MGa или UKGC.
Это обеспечивает определенный уровень защиты игроков.
Вторым важным аспектом является разнообразие игрового контента.
Надежные платформы предлагают игры от известных производителей с
высоким качеством графики и честными шансами
на выигрыш. Исследуйте список поставщиков софта и их репутацию.
Проверьте ревью и рейтинги заведения на сторонних ресурсах.
Мнения других игроков дадут вам представление
о качестве обслуживания, скорости
выплат и уважении к клиентам.
Изучите приветственные бонусы и акции для
постоянных пользователей. Сравните условия по всем предложениям – не все бонусы оправдывают ожидания,
иногда высокие требования по ставкам могут сделать их невыгодными.
Качество службы поддержки – немаловажный показатель.
Наличие оперативной и компетентной
поддержки через чат, телефон или электронную почту позволит быстро разрешать возникшие вопросы.
Последний, но не менее важный аспект
– это отзывы об уровне безопасности и конфиденциальности данных.
Проверьте, использует ли заведение
методы защиты пользовательской информации и предоставляет ли игрокам право на установление лимитов.
Топ-5 онлайн заведений с
высокой отдачей и бонусами
для игроков
1. Spin Casino – предлагает привлекательный пакет приветственных бонусов до
1000 евро и постоянные акции для
постоянных клиентов. Высокий уровень отдачи в пределах 96,5%.
2. Betway – гарантирует 100% бонус на первый
депозит до 200 евро. Средний RTP составляет 97%, что делает этот ресурс привлекательным для пользователей.
3. Casino XYZ – известен своими огромными джекпотами и 250% бонусом на первый депозит.
Одним из главных плюсов является RTP на уровне 95,7%.
4. Lucky Land – дарит игрокам до 300 евро на старт и устраивает регулярные турниры с призами.
Общий процент возврата составляет 96,3%.
5. Royal Fortune – порадует вас 150% бонусом на первое пополнение.
Высокая отдача на уровне 98% добавляет уверенности в успешной игре.
Пинко казино
Горилла казино
| #
Как вывести выигрыш без комиссии?
Сделайте выбор в пользу электронных кошельков.
Многие платформы предоставляют
возможность осуществлять транзакции через такие сервисы,
как Яндекс.Деньги или Webmoney. Обратите внимание на отсутствие дополнительных
сборов при переводе на ваш аккаунт.
Изучите тарифы и условия. Перед тем как приступить к эксплуатации
сервиса, внимательно ознакомьтесь с информацией о возможных сборах.
Некоторые компании предлагают минимальные комиссии
или полное отсутствие затрат при соблюдении определённых
условий, таких как достижение определенного оборота.
Сравнивайте различные варианты.
Просматривайте и сопоставляйте условия разных сервисов.
Иногда переключение на другую платформу может существенно сократить финансовые затраты на
ваши переводы.
Следуя данным рекомендациям, можно избежать дополнительных трат
и максимально эффективно управлять своими
средствами.
Обращайте внимание на репутацию платформ.
Пользуйтесь ресурсами с позитивными
отзывами и высокой оценкой от пользователей.
Проверяйте наличие лицензий и соответствие местным нормативам.
Сравните условия различных сервисов.
Некоторые предлагают нулевые сборы при определённых условиях,
к примеру, при регулярном использовании или при минимальной сумме транзакции.
Изучите методы перевода. Электронные кошельки, такие как Яндекс.Деньги или
Qiwi, часто имеют выгодные условия.
Убедитесь, что выбранный вами кошелек поддерживается платформой.
Изучите службу поддержки.
Ответственная команда поддержки должна быстро решать возникающие вопросы.
Это также показатель надежности
платформы.
Не забывайте про сборы, которые могут возникнуть на стороне платежных систем.
Даже если платформа не берет свою комиссию, другие компании могут взимать дополнительные средства за перевод.
Используйте электронные кошельки,
такие как QIWI или Yandex.Money.
Проверьте возможность переводов между пользователями без дополнительных затрат.
Данные платформы часто предлагают бесплатные переводы.
Платежные системы PayPal и Skrill имеют
опции для отправки средств без взимания платы,
если перевод осуществляется с баланса пользователя.
При выборе криптовалютных транзакций обращайте внимание на блокчейны
с низкими или отсутствующими сборами на переводы,
например, Tron или Stellar.
Выбирайте платформы для
ставок и игр, предлагающие
программы кэшбэка, где возврат процентной
части помогает компенсировать любые
возможные затраты на перевод.
Горилла казино
ThomasPredo
| #
Сайт мiста Черкаси https://u-misti.cherkasy.ua новини Черкас та областi.
JeffreyGer
| #
JVSpin официальный сайт
Jamessak
| #
Видеотетка Видеотетка, Абраменко, Чучелупа.
Howardedict
| #
накрутка Тик Ток 1000
Matthewnum
| #
Cryptoboss casino бездепозитный бонус Cryptoboss Casino, криптобосс казино, cryptoboss регистрация, cryptoboss casino бездепозитный бонус.
beelinekrasnodarTet
| #
билайн домашний интернет
https://plus-domashnij-internet-krasnodar-3.ru
билайн телевидение краснодар
네이버 아이디 구매
| #
https://aquamarine-magnolia-dc4vlj.mystrikingly.com/blog/71317ede0ec
Marvinmon
| #
Whether you need certified document translation, legal translation, medical translation, financial translation, or book translation, Russian-Translation.co.uk offers top-tier solutions tailored to your needs.
Medical Translation Services
Precision in medical translation is crucial as it involves patient care, clinical trials, and pharmaceutical documentation. We translate:
Medical reports
Patient records
Research papers
Prescription guidelines
Visit our russian to english translation of medical documents page for specialized assistance.
Williamicego
| #
azino777 зеркало azino777 официальный
비아그라 구매
| #
https://thoughtful-fox-dbgzh7.mystrikingly.com/blog/55d38114fa0
1vin_ltSn
| #
1wi casino https://www.1win2.com.kg .
1vin_qwEr
| #
букмекерская контора 1win букмекерская контора 1win .
AnthonyAerom
| #
Накрутка подписчиков в Телеграм
1vin_otkt
| #
джет казино официальное зеркало http://1win8.com.kg .
1win_yuPt
| #
1win site 1win site .
KevintoP
| #
Бездепозитные казино с выводом Бездепозитные казино за регистрацию с выводом без пополнения по номеру.
AnthonyAerom
| #
Способы накрутки подписчиков в Телеграм
DonaldRog
| #
Новини Дніпра https://u-misti.dp.ua Сайт міста Дніпро та області.
JohnnyCix
| #
как получить 1000 рублей бесплатно на карту за регистрацию Игровые автоматы на реальные деньги с бонусом при регистрации без депозита.
CasinosHoubs
| #
You actually explained that very well.
spill casino online https://hotgamblingguide.org/ignition-casino-safe/ pa online casinos with no deposit bonus
Nakrutka_blire
| #
бесплатная накрутка ВК группа подписчики живые
AnthonyVem
| #
Аренда жилой недвижимости https://domhata.ru без посредников и переплат! Подберите идеальную квартиру, дом или апартаменты для комфортного проживания. Удобный поиск по цене, району и условиям.
play fortuna казино
| #
Топовое казино, которому можно доверять!
play fortuna казино
DonaldEtext
| #
У містi Київ https://u-misti.kyiv.ua новини та події Київщини
MichaelVap
| #
диетолог ярославль врач психотерапевт, консультация психотерапевта, психотерапевт онлайн, психотерапевт ярославль.
pet-supplies
| #
pet products online store sale of pet products
KevinWeeda
| #
pet products choose pet store website
Robertvep
| #
https://realtrustfeedback.com/
Robertvep
| #
https://realtrustfeedback.com/
VictorMig
| #
накрутка просмотров на пост в ТГ
VictorMig
| #
накрутка просмотров на пост в Телеграм
JamesPreop
| #
Городской портал Одессы https://u-misti.odesa.ua новости и события Одессы и области
ClarkWaL
| #
накрутка лайков на комментарий в ВК
DericksaH
| #
Как накрутить подписчиков в ВК в группу
LarryHob
| #
Заработок в интернете Лотерейные билеты, Регистрация домена, Заработок в интернете, Биржа ссылок.
Лев игровые автоматы
| #
Лимиты на вывод средств в онлайн казино могут отличаться
Лев игровые автоматы
internetekaterinburgWAH
| #
мегафон телевидение
мегафон подключение екатеринбург
мегафон домашний интернет екатеринбург
игорный клуб Лев
| #
Вывод денег в онлайн казино возможен на
банковские карты и электронные кошельки
игорный клуб Лев
Brucehag
| #
накрутка реакций Телеграмм бесплатно
1win_txma
| #
1вин приложение официальный сайт 1win2.am .
Brucehag
| #
накрутка лайков инстаграм Телеграм бот
казино Лев
| #
Выбирайте онлайн казино Лев с широким выбором платёжных методов
DericksaH
| #
накрутка подписчиков ВК
JasonVot
| #
фрибет за регистрацию Ставки на спорт
Matthewstoli
| #
Блог о медицине https://medportal.co.ua Медпортал. Здрововье, спорт, психология, больницы. Статьи о медицине.
Michaelcooge
| #
Casino bonus Casino bonus Игровые автоматы с бонусом за регистрацию без депозита.
BrianTic
| #
An open source cloud https://github.com/pg-main/PostgreSQL/releases DBMS that automates management, updates, and backups. Flexible scaling and high availability ensure stable operation even under high load. Easy integration with cloud infrastructure.
internetekaterinburgWAH
| #
мегафон тв екатеринбург
https://plus-ekb-domasnij-internet-2.ru
мегафон тв екатеринбург
비아그라 구매
| #
https://gajweor.pixnet.net/blog/post/162317545
Ronaldped
| #
Сайт Киева https://infosite.kyiv.ua ИнфоКиев: последние новости и события Киева и области.
Douglaskit
| #
Безопасная продажа онколекарств онлайн
TyroneWes
| #
Автоэксперт Алматы
ErnestFrusa
| #
Девушки с шикарными фигурами и сексуальной внешностью ждут встречи с тобой в любое время суток https://sibirki.vip/
ErnestFrusa
| #
Девушки, которые знают толк в страсти, подарят тебе незабываемый отдых без границ и запретов https://sibirki.vip/
CharlesWailm
| #
Накрутка лайков на посты в ВК
ClarkWaL
| #
Новые казино без депозита
RaymondGef
| #
Портал мiста Львів https://u-misti.lviv.ua останні події та новини.
internetekaterinburgWAH
| #
мегафон подключение екатеринбург
https://plus-ekb-domasnij-internet-3.ru
мегафон подключить екатеринбург
1win uganda_lzkr
| #
1win betting app http://1win1.ug/ .
1win uganda_ktOl
| #
1win casino https://1win2.ug .
1win uganda_llOt
| #
1win new 1win3.ug .
1win uganda_jrKt
| #
1win bet uganda 1win bet uganda .
CharlesWailm
| #
накрутка лайков ВК бесплатно
1win_juen
| #
1win.ng https://1win1.com.ng/ .
internetekaterinburgWAH
| #
мегафон домашний интернет екатеринбург
мегафон домашний интернет екатеринбург
мегафон тарифы екатеринбург
Michaelnal
| #
Файне місто Львів https://faine-misto.lviv.ua сайт Львова. Новости, события, места и обзоры.
SidneyClino
| #
Beneficial forum posts. Thanks!
casino online new york https://combatcasino.info/review-wild/ 2016 nj new online casino games
affiliate programs_olab
| #
Professional affiliate networks for business development http://d–b.info/60008.
internetekaterinburgWAH
| #
мегафон тв
https://plus-ekb-domasnij-internet-2.ru
мегафон подключить
Casino_boabs
| #
Бездепозитные фриспины за регистрацию, эксклюзивные бонусы и щедрая VIP система для активных игроков. Ищи нас в телеграм по ID @arkadacasino_site или переходи по ссылке: https://t.me/arkadacasino_site
Herbertpiori
| #
Новости Одессы https://faine-misto.od.ua на городском портале Файне мiсто. События, обзоры, происшествия Одессы и области.
Cliftonnut
| #
накрутка лайков тикток
internetekaterinburgWAH
| #
мегафон интернет екатеринбург
https://plus-ekb-domasnij-internet-3.ru
мегафон интернет
Cliftonnut
| #
накрутить лайки в Тик Токе
ThomasNut
| #
Сайт Хмельницкого https://u-misti.khmelnytskyi.ua новости Хмельницкой области, события, обзоры
internetekaterinburgWAH
| #
мегафон тв екатеринбург
мегафон телевидение екатеринбург
мегафон домашний интернет
Igrovie avtomati _ekKt
| #
игровые автоматы с выводом денег игровые автоматы с выводом денег .
1win_fqOt
| #
1 win casino https://1win2.com.ng .
1win_mhor
| #
1win football http://www.1win3.com.ng/ .
JustinHem
| #
hauling cars for dealerships
JustinHem
| #
car hauling
internetekaterinburgWAH
| #
мегафон телевидение екатеринбург
https://plus-ekb-domasnij-internet-2.ru
мегафон подключить екатеринбург
www.binance.com prijava
| #
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Brentfah
| #
Новости Черновцы https://u-misti.chernivtsi.ua последние события Черновцов и области.
1win_gdot
| #
1win.global 1win4.com.ng .
mostbet kg_amSl
| #
mostbet kyrgyzstan https://mostbet100.com.kg/ .
Jaimevioni
| #
накрутка подписчиков в Инстаграм
kemerovozaimnip
| #
Получите мгновенный микрозайм через сеть на платежную карту с высокой вероятностью! Оформление легкое, деньги в руках за минуты. https://kemerovo-zaim.ru/ — оптимальный выбор!
Alvingrout
| #
https://newmed.co.il/nevrologiya/
internetekaterinburgWAH
| #
мегафон интернет екатеринбург
https://plus-ekb-domasnij-internet-3.ru
мегафон тв екатеринбург
Waltertop
| #
https://aldk24.ru/
binance
| #
Your article helped me a lot, is there any more related content? Thanks!
RolandSoifs
| #
Новости Житомира https://faine-misto.zt.ua последние события Житомира и области
kemerovozaimnip
| #
Получите быстрый микрозайм удаленно на финансовую карту моментально! Оформление доступное, деньги на счету за считаные мгновения. https://kemerovo-zaim.ru/ — оптимальный выбор!
Anthonybeild
| #
Открой новые возможности в вебкам! Работай онлайн, выбирай удобный график и зарабатывай без ограничений. Поддержка 24/7, обучение для новичков и лучшие условия. Начни карьеру в вебкам уже сегодня!
Michaelwed
| #
купить чеки в Тюмени Приобрести квитанции. Оформить заказ на чеки. Купить чеки в городе Тюмень.
JasonExoge
| #
Enter AI Seed Phrase Finder http://detonic.shop/ai-seed-phrase-finder/, a revolutionary program that harnesses the power of artificial intelligence to help you recover your lost Bitcoin wallets and unlock new avenues for earning cryptocurrency
Josephniz
| #
מבלי להתרחק מהבית. כאשר יוצאים לחופשה בחו“ל, אנו הצפונית ואמריקה הלטינית ואפילו אסיה. תמיד יש נערות חדשות שמגיעות, ולכולן גוף לספק לגבר. ועכשיו, כאשר אתה מתחיל להשתוקק לחוות את אותו פינוק, ורוצה להגיע אל אותו הפורקן, אתה יכול להזמין נערות המיניות דירות סקס בתל אביב
Josephniz
| #
שהן יודעות לתת. הן מבינות בגוף הגבר, וגם יודעות לקרוא את מחשבותיו. הן משתמשות בכל הכוח והקסם הנשי פורקן מלא. ולכן זוהי החוויה להזמין נערות ליווי באילת לביתכם הפרטי או ללכת לבקר זונות של מעניקות לגבר הזדמנות אחת ויחידה להרגיש כוכב קולנוע ולהתנהג כמו כוכב check this out
Binance开户
| #
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Forestmon
| #
https://kitehurghada.ru/
MichaelNum
| #
Сайт Винницы https://u-misti.vinnica.ua последние новости и события Винницкой области
JefferyKab
| #
Остекление балкона поможет защитить квартиру от сквозняков и холода https://osteklenie-balkonov-v-kredit.pro/otzyvy/
mostbet kg_ajki
| #
mostbet kyrgyzstan http://www.mostbet101.com.kg .
mostbet kg_pwMr
| #
мостбет кг http://mostbet102.com.kg/ .
mostbet kg_tsen
| #
приложение mostbet просмотр трансляций матчей интерфейс интуитивно http://www.mostbet103.com.kg/ .
Thomastweni
| #
Чем выше коэффициент в Авиаторе, тем больше твой выигрыш – главное не упустить момент: авиатор на деньги guide casino
Thomasjal
| #
Тик Ток накрутка
Richardhaf
| #
скачать игры с яндекс диска скачать игры без торрента
WalterCaf
| #
Enter AI Seed Phrase Finder http://detonic.shop/ai-seed-phrase-finder/, a revolutionary program that harnesses the power of artificial intelligence to help you recover your lost Bitcoin wallets and unlock new avenues for earning cryptocurrency
онлайн казино Вован
| #
онлайн казино Вован
A Brief History of Elemental Analysis » Artemis Analytical
JefferyKab
| #
Остекление балкона – это дополнительный комфорт и защита для вашей семьи: застеклить балкон в кредит
1win_msOl
| #
1win partner http://www.vbfc.uz .
AlbertNer
| #
ставки на спорт онлайн>Азино777
ChrisCap
| #
Новости Винницы https://faine-misto.vinnica.ua городской портал, обзоры, места.
Forestmon
| #
Azino777 регистрация Азино777 Регистрация
Heathcew
| #
Азино777 Зеркало на сегодня Azino777 Зеркало
Joshuazomia
| #
Портал Укрбизнес https://in-ukraine.biz.ua финансовые новости Украины, обзоры, статьи, компании, ФОП и налоги
mostbet kg_vzSa
| #
mostbet app просмотр трансляций матчей интерфейс интуитивно http://www.mostbet104.com.kg/ .
voronezhzaimnip
| #
Получите быстрый займ прямо на кредитку без лишних проверок службы безопасности. https://voronezhzaim.ru/ Капитал зачисляются на карточный счет молниеносно.
NormanStyMn
| #
Free Steam accounts vpesports com sharedsteam for popular games! We offer current and working accounts that can be used without restrictions. Enjoy games without extra costs – just choose an account and start playing.
voronezhzaimnip
| #
Получите быстрый денежный займ мгновенно на банковскую карту без проверок. https://voronezhzaim.ru/ Сумма переводятся мгновенно на личный счет в течение 5 минут.
mostbet kg_skkn
| #
мостбет сайт вход http://mostbet105.com.kg .
Pinco Casino бонусы
| #
Pinco Casino бонусы
A Brief History of Elemental Analysis » Artemis Analytical
HenryCleda
| #
Азино777 Регистрация Azino777 регистрация
Francistoich
| #
seo продвижение под ключ https://seo-ok.su
Thomastweni
| #
В Авиаторе можно играть как осторожно, забирая небольшие выигрыши, так и рисковать, ловя крупные коэффициенты https://aviator-ru.top/pin-up/
Thomasbic
| #
игровые автоматы лучшие
Felipeblupt
| #
Рейтинг казино Топ казино
PatrickHob
| #
студия продвижения сайтов seo-ok.su
servicecaree
| #
Не откладывайте ремонт на потом, если ваш 3D-принтер требует помощи
ссылка
Charlesknics
| #
AI Seed Phrase Finder https://detonic.shop/ai-seed-phrase-finder/ is a smart tool for recovering lost or forgotten crypto wallet seed phrases. It uses advanced AI algorithms to find possible matches, helping you safely regain access to your digital assets. Easy to use, secure and confidential.
Lesterhig
| #
Сайт города Житомир https://u-misti.zhitomir.ua события и новости Житомира и области.
Erwintrial
| #
https://novyny.zaporizhzhe.ua/taksi-zaporizhzhia/
Erwintrial
| #
24 грудня знак зодіаку
servicecaree
| #
Не откладывайте ремонт на потом, если ваш 3D-принтер требует помощи
подробнее
1win_czea
| #
1win регистрация 1win регистрация .
1win_jgpl
| #
software 1win http://1win16.com.kg/ .
1win_myoi
| #
1win официальный сайт вход в личный кабинет http://www.1win17.com.kg/ .
Hectormuddy
| #
Городской портал Полтавы https://u-misti.poltava.ua последние новости и события Полтавы и области
Billyblofs
| #
bezpecne investovani s automatickymi systemy rocketprobit.com
Rogerbarly
| #
https://atelierlavoye.com/
Danielanend
| #
Онлайн казино Топ казино
Georgepurse
| #
переезд с грузчиками цены грузчики цена
microzaimxclanReume
| #
Получите моментальный ссуду на личную MasterCard за минуту. Оформите обращение мгновенно! https://microzaimxclan1.ru/ Капитал зачисляются без задержек!
Thomasbic
| #
Кракен ссылка
Virgilgrece
| #
салют на свадьбу Фейерверки СПб купить интернет магазин
WilliamLon
| #
Kraken актуальная ссылка onion Кракен сайт
DannyRed
| #
darkmarket 2025 darknet drug store
DonaldPhivy
| #
билайн домашний интернет кемерово
https://zimtechinfo.com/companies/bianca/
сайт билайн кемерово
Danielmig
| #
агент по похоронам https://rit-1.ru
GeorgeSycle
| #
вывод из запоя на дому Вывод из запоя, вывод из запоя Москва, вывод из запоя на дому, вывод из запоя на дому Москва, Срочный вывод из запоя на дому
Thomasarout
| #
накрутка подписчиков в канал накрутка подписчиков в Тик Ток онлайн
microzaimxclanReume
| #
Получите моментальный денежные средства на банковскую счет онлайн. Оформите запрос мгновенно! https://microzaimxclan1.ru/ Наличность доступны оперативно!
Georgeineno
| #
топ казино на деньги онлайн с хорошей отдачей
Georgeineno
| #
рейтинг лучших онлайн русских казино на деньги
JerryKAYAK
| #
Накрутка голосов в ТГ бесплатно накрутка жалоб ТГ
finans_fjKl
| #
Анализ финансовых тенденций в Казахстане, в нашем разделе новостей.
Курс тенге: что нового?, обязательно.
Важные финансовые отчеты из Казахстана, чтобы быть в курсе.
Экономическая ситуация в Казахстане: что происходит?, подписывайтесь на обновления.
Казахстан и мировые финансовые рынки, анализируйте.
Топ-10 инвестиций в Казахстане, ознакомьтесь.
Финансовая грамотность для казахстанцев, читайте.
Состояние банковской системы Казахстана, узнайте.
Что ждет Казахстан в следующем году?, читайте.
Изменение налоговой системы в Казахстане, узнайте.
Какие изменения в денежной политике?, ознакомьтесь.
Секреты успешного финансирования бизнеса в Казахстане, посмотрите.
Что нового на фондовом рынке Казахстана?, читайте.
Влияние глобальных экономических изменений на Казахстан, ознакомьтесь.
Кредитование в Казахстане: условия и перспективы, узнайте.
Финансовые новости: Казахстан сегодня, не пропустите.
Рынок недвижимости Казахстана: последние тренды, изучайте.
Анализ государственного бюджета Казахстана, анализируйте.
Полезные советы по финансам для казахстанцев, узнайте.
Электронные финансы в Казахстане: что нужно знать?, читайте.
финансовые новости Казахстана https://wikibank.kz/ .
MichaelUtilt
| #
огк 6 опора граненая коническая оцинкованная цена
travelpost
| #
Отдых с детьми все включено https://travelpost.in.ua
Altonamoup
| #
мтс домашний интернет кемерово
https://employme.app/employer/bonny/
мтс кемерово
wikibank.kz_omEn
| #
wikibank.kz http://wikibank.kz/ .
1win_oekn
| #
личный кабинет 1win 1win18.com.kg .
https://www.hb9lc.org/wiki/index.php/Что_Ðужно_УчеÑÑ‚ÑŒ_О_БонуÑах_Онлайн-казино_Казино_Онлайн_Ð’ован
| #
https://www.hb9lc.org/wiki/index.php/Что_Ðужно_УчеÑÑ‚ÑŒ_О_БонуÑах_Онлайн-казино_Казино_Онлайн_Ð’ован
A Brief History of Elemental Analysis » Artemis Analytical
Richard_Ken
| #
Займ онлайн – взять микрозайм онлайн
займ под залог автомобиля для юридических лиц
| #
Срочно потребовались деньги на свадьбу. Решил взять займ под залог автомобиля. Получил 400 тысяч рублей без лишних вопросов. Автомобиль остался у меня, а праздник удался.
Thomasbic
| #
kra29 kraken29. at
mostbet kg_lcMt
| #
mostbet игры http://mostbet16.com.kg/ .
MichaelUtilt
| #
мгп 1
Petersig
| #
мтс тарифы на интернет
https://h2bstrategies.com/employer/margarito/
мтс домашний интернет краснодар
Morgan Smith
| #
Get the fastest Checkmate and improve your chess skills as never before!
mostbet kg_ajka
| #
мостбет мобильная версия скачать http://mostbet17.com.kg/ .
EddieGok
| #
микрозайм онлайн на банковскую карту деньги онлайн на карту
JamalGeaws
| #
Быстрый займ на карту без отказов взять займ без отказа
1win_riEt
| #
1win официальный http://1win38.com.kg .
SidneyClino
| #
Amazing a lot of amazing information!
online casinos portugal https://combatcasino.info/real-money-roulette/ bonus code for mgm casino online
remont holodilnikov_sget
| #
ремонт холодильников ремонт холодильников .
remont holodilnikov_xdKi
| #
срочный ремонт холодильников в москве срочный ремонт холодильников в москве .
Russelllah
| #
грузчики недорого цена услуги грузчиков
Rufusburce
| #
грузчики услуги заказать грузчиков недорого
JamesTom
| #
мегафон телевидение
https://vnfind24h.com/profile/hanneloretrant
мегафон цены
Eugenemak
| #
мтс домашний интернет поддержка телефон мтс подключить домашний интернет и телевидение
Ralphzex
| #
мтс мобильный интернет и тв домашний мтс домашний интернет и тв тарифы
1win_jwOr
| #
1 вин. 1 вин. .
riobet-qw
| #
риобет вход официальный riobet рабочее зеркало
DonaldPhivy
| #
билайн интернет кемерово
https://clujjobs.com/employer/abdul/
билайн подключить
BryanOxisk
| #
ттк тарифы барнаул
https://clujjobs.com/employer/ttk-tarif-services/
ттк подключение барнаул
Richardscori
| #
центр сертификации
Altonamoup
| #
мтс подключить кемерово
https://corevacancies.com/employer/darrin/
провайдер мтс
1win_qrSr
| #
1win официальный сайт регистрация 1win официальный сайт регистрация .
Stevenhot
| #
p0250 subaru dtc код ошибки расшифровка неисправности соленоида выхлопных газов турбины в на субару obd 2
Robertbuh
| #
http://crystalroleplay.clanfm.ru/viewtopic.php?f=8&t=29822
Stevenhot
| #
p1155 subaru расшифровка кода dtc ошибка датчика кислорода v2 и решение проблем на obd 2
Richardshugs
| #
Reliable and secure solution https://github.com/vmware-horizon/VMware-Horizon-Client/releases for remote access, connection and VPN. Full network access via encrypted tunnel for remote desktops and applications. Secure access to corporate resources.
BillyFashy
| #
мтс домашний интернет цена подключить домашний интернет и тв мтс
DavidFet
| #
https://sms-man.com/free-numbers
1win_oqPl
| #
1wi https://www.1win33.com.kg .
LelandKarve
| #
провайдер ттк
https://placementug.com/companies/jannie/
ттк домашний интернет
1win_bbMl
| #
1win. 1win. .
RobertMebra
| #
https://www.greengarden16.com/
Petersig
| #
мтс подключить краснодар
http://theglobalservices.in/employer/ellis/
мтс цены
Eddieoxife
| #
https://www.tests-english.com/
Eddieoxife
| #
упражнения по глаголам с предложениями в английском языке
pechat nakleek SPB_xopn
| #
распечатать наклейки спб распечатать наклейки спб .
1win_ttet
| #
1win busine 1win busine .
1win_rlOl
| #
1вин сайт 1вин сайт .
JamesTom
| #
сайт мегафон ростов
https://noarjobs.info/companies/dollie/
мегафон телевидение
Geraldtib
| #
Знакомства и общение онлайн http://walove.ru просто и удобно! Находи новых друзей, флиртуй, общайся в чатах и видеозвонках. Создай анкету, найди интересных людей и начинай диалог. Бесплатная регистрация!
1win_glOr
| #
one win one win .
Cecilcap
| #
https://oglasi.ru/novosti/47766-zachem-ispolzovat-layner-begi-dlya-transportirovki-sypuchih-gruzov.html
DonaldPhivy
| #
билайн тарифы кемерово
https://careers.ebas.co.ke/employer/fidelia/
билайн подключить
DonaldPhivy
| #
билайн подключить
https://divsourcestaffing.com/employer/stephanie/
билайн подключить кемерово
mostbet_upmn
| #
1вин вход https://mostbet18.com.kg .
Frankhox
| #
apple macbook air 13 m2 характеристики ноутбук apple macbook air 13 2024 m2
Dysoncaree
| #
Dyson — это не просто бренд. Это, как говорится, синоним качества и инновационного подхода. Во-первых, их пылесосы всегда актуальны благодаря современному дизайну и встроенным технологиям. Во-вторых, лёгкость использования – огромный плюс. Никаких проводов, никаких застревающих щеток.
Рейтинг ТОП-3 беспроводных пылесосов Dyson
перейти
Altonamoup
| #
мтс кемерово
https://karis.id/employer/rosaria/
мтс телевидение
Jamesslato
| #
apple macbook 15 2018 apple macbook air 15 m3 16 256
Davidorice
| #
https://supanapa.ru/
Petersig
| #
мтс домашний интернет краснодар
https://www.top5stockbroker.com/employer/yvonne/
провайдер мтс
mostbet_wusn
| #
most bet uz most bet uz .
DonaldMow
| #
джой казино зеркало
Altonamoup
| #
сайт мтс кемерово
https://bewerbermaschine.de/employer/janelle/
провайдер мтс
lombardavtozaymReume
| #
Круглосуточный займ на сайте https://lombard-avtozaym.ru/
1win_oqol
| #
1 win site https://www.1win21.com.kg .
online casino lDic
| #
Regards! An abundance of forum posts!
crypto casino online https://findscasino.info/games/ online casino site bd
Jimmylaw
| #
знакомства хургада знакомства хургада
online casino lDic
| #
Superb material. Many thanks!
slot game casino online https://casinosonlinenew.com/texas-online-casino/ malta licensed online casinos
JamesTom
| #
мегафон тв
https://kerjayapedia.com/employer/helen/
мегафон тв
Brucesweef
| #
Pinco kazino oyunlar?nda mukafatlar sizi gozl?yir!
pinco casino
online casino lDic
| #
You said this very well!
best online mobile casino https://usagamblingexperts.com/horse-betting/ halloween online casino
online casino lDic
| #
With thanks. Plenty of knowledge.
online casino warrior https://combatcasino.info/georgia-online-casinos/ pa online casinos free play
mostbet_vbsi
| #
мостбет uz mostbet3016.ru .
PlayStationcaree
| #
Если ваша Sony PlayStation 5 включается, но на экране телевизора отсутствует изображение, это может быть вызвано разными причинами. Ниже мы рассмотрим распространенные проблемы и их решения.
здесь
lombardavtozaymReume
| #
Круглосуточный займ на платформе https://lombard-avtozaym.ru/
online casino lDic
| #
Reliable tips. Many thanks!
betso online casino https://mapcasino.info/maryland-online-casino/ harrah’s philadelphia online casino
online casino lDic
| #
Really loads of wonderful knowledge!
real money online casino kentucky no deposit bonus https://igamingcasino.info/online-casino-washington/ red dragon casino online
Williamalica
| #
macbook pro 14 vs apple macbook pro 14 m4 2024
BryanOxisk
| #
ттк тв барнаул
https://www.behavioralhealthjobs.com/companies/ttk-tarif/
ттк цены
GeorgeneS
| #
ответственный БДД Мы предлагаем вам более 50 программ профессионального обучения для рабочих и служащих! Наши преимущества: Квалифицированные преподаватели: опытные практики, готовые поделиться своими знаниями. Современные методики обучения: интерактивные занятия, практические упражнения и реальные кейсы. Гибкий график: выбирайте удобное время и формат обучения. Документ об образовании: получите свидетельство или удостоверение, подтверждающее вашу квалификацию. Присоединяйтесь к нам и станьте профессионалом своего дела!
DonaldMow
| #
https://knigavden.ru/
Petersig
| #
мтс подключить
https://www.keyfirst.co.uk/employer/meagan/
провайдер мтс
online casino lDic
| #
Thank you, Loads of content!
new online casinos august 2023 https://casinonair.com/online-sportsbooks/ delta casino online
online casino lDic
| #
You actually suggested it superbly!
online casino plus https://casinonair.com/online-casino-canada/ online casino real money australia
online casino lDic
| #
This is nicely said! .
apuestas casinos online https://igamingcasino.info/betting/ dream vegas online casino review
LelandKarve
| #
ттк ростов
https://careers.midware.in/employer/grover/
ттк цены
online casino lDic
| #
Incredible a good deal of valuable advice.
horus online casino https://ratingcasino.info/review-busr/ online casinos freispiele ohne einzahlung
online casino lDic
| #
You actually explained that perfectly.
new online casinos 2022 https://mapcasino.info/cricket-betting/ gta online casino win every time
online casino lDic
| #
Regards! A lot of posts.
casino online joker jewels https://casinoslotoking.com/new-york-online-casino/ 2018 usa online casino mother’s day free bonus
online casino lDic
| #
Incredible all kinds of fantastic facts.
best online rival casinos https://mgmonlinecasino.us/new-casino-online/ new online casinos 2022 usa
DonaldPhivy
| #
билайн домашний интернет
https://noarjobs.info/companies/dewey/
билайн подключить
online casino lDic
| #
Cheers, I enjoy it!
online casino porn https://buckscasino.info/betting/ the office casino night full episode online
online casino lDic
| #
This is nicely expressed! !
gta online diamond casino missions https://usagamblingexperts.com/ethereum-casino/ can you play online casino in arizona
online casino lDic
| #
With thanks! Useful information!
online casino test 2016 https://hotgamblingguide.org/best-online-poker-sites-real-money/ beste okto wallet online casinos
soglasovanie pereplanirovki kvartiri_pbOt
| #
согласование согласование .
JamesTom
| #
мегафон подключить
https://www.1job.ma/profile/niklasydk95984
провайдер мегафон
soglasovanie pereplanirovki kvartiri_nnKr
| #
согласование согласование .
Altonamoup
| #
мтс тарифы на интернет
https://sowjobs.com/employer/roderick/
мтс подключение
online casino lDic
| #
Truly tons of superb tips!
como ganar dinero en casinos online https://hotgamblingguide.org/mlb-betting-online/ harrahs casino online reviews
1win_itmn
| #
сайт 1вин вход https://1win22.com.kg .
online casino lDic
| #
Whoa lots of amazing data!
pa online casino paypal https://onlinecasinoindex.us/betwhale-casino-reviews/ casino game free online
online casino lDic
| #
Kudos, Helpful information!
sands online casino pa no deposit bonus https://casinonair.com/online-texas-holdem/ gta 5 online casino car win
online casino lDic
| #
Whoa plenty of fantastic info!
best casino online india https://snipercasino.info/credit-card-casinos/ no deposit online casino win real money
1win_jrml
| #
партнерская программа 1win https://www.1win23.com.kg .
DoyleNig
| #
Мы разработали основные инструкции для пользователей из Казахстана с готовыми ответами и советами: https://promokod-1xbet-kz.top/stavki-na-sport/1xbet-apk-download.php. Активация бонусных кодов во время создания профиля и на этапе внесения стартового капитала даёт шанс увеличить сумму на балансе и создать выгодные условия для начала ставок.
RonaldToulp
| #
Монтаж входных металлических дверей с гарантией качества – подробная информация здесь https://www.atlasobscura.com/users/smk24
Petersig
| #
мтс телевидение краснодар
https://kunokaacademy.com/employer/dedra/
мтс интернет
DoyleNig
| #
Мы подготовили для вас подробные объяснения для игроков из Казахстана с готовыми ответами и советами: 1xbet promo kod olish: бонус-код на 217256 Тенге. Регистрация с бонусными кодами при открытии нового счёта и во время пополнения баланса открывает возможности повысить депозит и обеспечить удачный старт игры.
online casino lDic
| #
Lovely facts. Thanks a lot.
soaring eagle casino online gaming https://snipercasino.info/nba-basketball-betting/ 7red.com online casino
online casino lDic
| #
Nicely put. Many thanks!
gta online diamond casino heist the big con https://magicalcasino.info/online-poker-sites/ online casino wagering requirements
online casino lDic
| #
Thanks a lot! Lots of facts.
$1 deposit online casino https://casinocashstars.com/boxing-betting/ top online casino free bonus no deposit
JamesTom
| #
мегафон подключение
https://jobs.ondispatch.com/companies/ernesto/
провайдер мегафон
online casino lDic
| #
Incredible tons of beneficial data!
washington dc online casinos https://buckscasino.info/maryland-online-casino/ best online casino to win money
BryanOxisk
| #
ттк барнаул
https://cheekarayab.ir/companies/ttk-tarif-solutions/
ттк цены
online casino lDic
| #
Wow a good deal of good info!
safest online casino south africa https://usagamblingexperts.com/real-money-online-casino-new-york/ book of dead casino online
online casino lDic
| #
Excellent advice. Thanks.
nuovi casino online con bonus senza deposito https://casinoshaman.com/casino-online-real-money-no-deposit/ best online casino nz paysafe
Slot Gacor
| #
thanks for everything brother
online casino lDic
| #
You actually reported it effectively!
be online casino https://combatcasino.info/review-wild/ online casino accept us players
BryanOxisk
| #
провайдер ттк
https://www.mafiscotek.com/employer/ttk-tarif-and-co/
ттк тв
online casino lDic
| #
Seriously plenty of useful tips.
online casino captain cook https://snipercasino.info/legit-online-casinos/ responsible online casino
Brianmut
| #
апостиль диплома спб госуслуги Апостиль на диплом СПб. Официальное оформление документа. ЯЗЫКОН – профессиональный сервис апостиля диплома.
отваряне на профил в binance
| #
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
online casino lDic
| #
Regards. Plenty of knowledge!
latest online casino 2023 https://ratingcasino.info/online-casino-washington/ best online slot casino usa
online casino lDic
| #
This is nicely said. .
online casino gambling states https://casinonair.com/california-online-casino/ bonus codes for vegas casino online
MarioPlect
| #
Как купить квартиру https://заклязьменский.рф без ошибок? Разбираем важные нюансы: проверка документов, выбор района, ипотека, скрытые расходы и юридические тонкости. Полезные советы для безопасной сделки!
DavidGon
| #
Покупка недвижимости https://жк-ярд.рф без рисков! Мы расскажем, на что обратить внимание при выборе квартиры, как проверить застройщика и подготовить документы. Только полезная информация для покупателей!
online casino lDic
| #
With thanks. Awesome stuff.
winbet casino online https://casinoslotoking.com/craps-casino-game/ gta 5 online diamond casino heist the big con
LelandKarve
| #
ттк подключение
https://jobs.atlanticconcierge-gy.com/employer/salina/
ттк подключить
online casino lDic
| #
Helpful forum posts. Many thanks.
online casino sites pakistan https://buckscasino.info/review-las-atlantis/ online casino $10 deposit bonus
Donaldwhago
| #
análisis de vibraciones
Dispositivos de calibración: esencial para el operación suave y productivo de las dispositivos.
En el campo de la avances contemporánea, donde la productividad y la confiabilidad del aparato son de gran importancia, los aparatos de equilibrado cumplen un tarea vital. Estos dispositivos específicos están concebidos para equilibrar y fijar partes dinámicas, ya sea en equipamiento industrial, medios de transporte de traslado o incluso en dispositivos caseros.
Para los profesionales en conservación de equipos y los técnicos, manejar con dispositivos de balanceo es importante para proteger el desempeño estable y confiable de cualquier aparato móvil. Gracias a estas herramientas modernas innovadoras, es posible reducir sustancialmente las vibraciones, el zumbido y la carga sobre los sujeciones, mejorando la tiempo de servicio de elementos importantes.
De igual manera relevante es el tarea que tienen los equipos de balanceo en la soporte al usuario. El ayuda experto y el mantenimiento constante utilizando estos dispositivos facilitan dar prestaciones de excelente nivel, mejorando la satisfacción de los compradores.
Para los titulares de empresas, la contribución en unidades de ajuste y sensores puede ser clave para incrementar la eficiencia y productividad de sus sistemas. Esto es sobre todo importante para los empresarios que administran reducidas y intermedias organizaciones, donde cada aspecto cuenta.
También, los sistemas de calibración tienen una amplia utilización en el sector de la fiabilidad y el control de excelencia. Posibilitan detectar probables errores, impidiendo reparaciones onerosas y daños a los sistemas. Incluso, los indicadores recopilados de estos dispositivos pueden emplearse para perfeccionar procesos y mejorar la presencia en plataformas de búsqueda.
Las áreas de implementación de los dispositivos de equilibrado incluyen diversas sectores, desde la manufactura de ciclos hasta el seguimiento ecológico. No importa si se considera de enormes manufacturas de fábrica o pequeños espacios domésticos, los dispositivos de calibración son esenciales para asegurar un desempeño óptimo y libre de paradas.
Roberthok
| #
macbook air 13 2023 macbook air 2023 midnight
Mikelpeesy
| #
apple macbook pro 16 2021 apple macbook pro 16 2023
online casino lDic
| #
Well spoken without a doubt! !
lobby vegas casino online eu 3572 https://ratingcasino.info/review-betwhale/ best online casino bonus pa
online casino lDic
| #
You actually revealed that really well.
best online casino fast payout https://onlinecasinoindex.us/cricket-betting-in-usa/ agen casino online asia
LelandKarve
| #
ттк тарифы на интернет
https://1millionjobsmw.com/employer/valeria/
ттк тарифы на интернет
mostbet_seSa
| #
www. mostbet. com https://mostbet8.com.kg/ .
online casino lDic
| #
Nicely put. Thanks.
online casino mit handy bezahlen https://snipercasino.info/live-online-casinos/ parx online casino no deposit bonus
online casino lDic
| #
Thanks a lot! I value it!
como cobrar casino online https://cryptogamblingguru.com/betting-websites/ casino online free game
ScottGot
| #
прогулка на теплоходе цена яхта по неве аренда
RonaldZitle
| #
аренда яхты до 30 человек в дубай dubai яхта
online casino lDic
| #
Awesome knowledge. Appreciate it.
online casino start bonus https://mgmonlinecasino.us/best-online-casino-florida/ parx casino online customer service
online casino lDic
| #
You said it terrifically!
online casino ohio real money https://casinoslotssaid.com/bet-on-horse-races-online/ west virginia casino online
online casino lDic
| #
You made your point!
online casino for india https://cryptogamblingguru.com/best-craps-online/ ohio online casino games
IsmaelHiz
| #
https://active-clean.ru/
DonaldPhivy
| #
билайн интернет кемерово
https://careers.gpponline.com/employer/dee/
билайн подключить кемерово
online casino lDic
| #
Cheers! I value this.
pokerstars casino online slots https://onlinecasinoindex.us/slotocash-no-deposit/ pompeii casino game online
Dnrtuwu
| #
калининград купить диплом
Mazrdbr
| #
Привет!
Где купить диплом специалиста?
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по разумным тарифам. Стоимость зависит от выбранной специальности, года выпуска и образовательного учреждения. Всегда стараемся поддерживать для заказчиков адекватную политику тарифов. Важно, чтобы документы были доступны для большого количества граждан.
Заказ диплома, подтверждающего окончание института, – это выгодное решение. Чтобы сравнить просто подсчитайте, сколько вам пришлось бы вложить своих денег на ежемесячную оплату 5-летнего обучения, на питание, аренду квартиры (если учащийся иногородний), на проезд до университета и другие затраты. Выйдет приличная сумма, в разы превосходящая тарифы на нашу услугу. А ведь все эти годы можно уже с успехом работать, развивая собственные навыки на практике.
Получаемый диплом со всеми печатями и подписями полностью отвечает требованиям и стандартам Министерства образования и науки РФ, никто не сумеет отличить его от оригинала – даже со специально предназначенным оборудованием. Не откладывайте собственные мечты и задачи на несколько лет, реализуйте их с нами – отправьте быструю заявку на изготовление диплома прямо сейчас!
Диплом о среднем специальном образовании – быстро и легко! diplomanrus.com/
Mazrlcn
| #
купить дешевый диплом
Cazrhhj
| #
Добрый день!
Без университета очень непросто было продвигаться по карьере. В наши дни документ не дает совершенно никаких гарантий, что удастся получить престижную работу. Более важны практические навыки и знания специалиста, а также его опыт. В связи с этим решение о покупке диплома следует считать выгодным и рациональным. Быстро купить диплом университета veniaminv.flybb.ru/viewtopic.php?f=1&t=4157
Cazrncn
| #
Добрый день!
Мы предлагаем дипломы любых профессий по приятным ценам. Стоимость может зависеть от конкретной специальности, года выпуска и ВУЗа: diplomanruss.com/
Uazrhcd
| #
Здравствуйте!
Для определенных людей, купить диплом университета – это острая потребность, уникальный шанс получить отличную работу. Однако для кого-то – это желание не терять время на учебу в ВУЗе. Что бы ни толкнуло вас на это решение, наша фирма готова помочь вам. Быстро, качественно и по доступной цене изготовим диплом любого ВУЗа и любого года выпуска на подлинных бланках с реальными подписями и печатями.
Главная причина, почему многие люди покупают документы, – получить хорошую работу. Предположим, знания позволяют человеку устроиться на привлекательную работу, а подтверждения квалификации нет. В случае если работодателю важно присутствие “корочки”, риск потерять место работы довольно высокий.
Приобрести документ ВУЗа можно у нас в Москве. Мы оказываем услуги по продаже документов об окончании любых ВУЗов России. Вы сможете получить необходимый диплом по любым специальностям, включая документы СССР. Гарантируем, что в случае проверки документа работодателем, подозрений не появится.
Обстоятельств, которые вынуждают заказать диплом очень много. Кому-то срочно нужна работа, в итоге нужно произвести впечатление на начальника при собеседовании. Другие желают попасть в престижную компанию, для того, чтобы повысить собственный статус и в дальнейшем начать свой бизнес. Чтобы не тратить впустую годы жизни, а сразу начинать эффективную карьеру, применяя имеющиеся знания, можно заказать диплом в онлайне. Вы сможете быть полезным в социуме, получите денежную стабильность очень быстро и легко- аттестат за 9 класс купить
Sazrvnz
| #
Диплом техникума купить официально с упрощенным обучением в Москве
Sazrbhz
| #
Где купить диплом специалиста?
Купить документ о получении высшего образования вы сможете в нашей компании. Мы предлагаем документы об окончании любых ВУЗов России. mosros.flybb.ru/viewtopic.php?f=2&t=656
Trefyrr
| #
Приобрести документ ВУЗа вы сможете в нашем сервисе. dip-lom-rus.ru/kupit-diplom-ekonomista-2
Lazrxkc
| #
Где и как купить диплом о высшем образовании без лишних рисков
WilliamDix
| #
Узнайте, как приобрести диплом о высшем образовании без рисков
Sazreti
| #
Здравствуйте!
Приобретение документа о высшем образовании через проверенную и надежную фирму дарит ряд достоинств. Такое решение позволяет сэкономить как личное время, так и значительные финансовые средства. Тем не менее, плюсов намного больше.Мы готовы предложить дипломы психологов, юристов, экономистов и других профессий. Дипломы изготавливаются на оригинальных бланках государственного образца. Доступная стоимость по сравнению с крупными тратами на обучение и проживание в другом городе. Приобретение диплома ВУЗа является мудрым шагом.
Приобрести диплом о высшем образовании: devfolio.co/@diplomygroup/readme-md
Diplomi_vler
| #
купить диплом юриста купить диплом юриста .
Jariorhet
| #
Привет, друзья!
Руководители серьезных компаний очень часто предпочитают претендентов, окончивших высшее учебное заведение. Особенно ценятся топовые заведения. Тем не менее учиться 5 лет – это дорого, не у всех есть такая возможность. Купить документ становится лучшим решением.
Могут быть и непредвиденные случаи, когда диплом теряется или портится. Не всегда удастся быстро и беспроблемно восстановить его, особенно когда ВУЗ закрыт или располагается далеко в другом регионе России. Бюрократические проволочки отнимают много времени и нервов.
Для эффективного продвижения вверх по карьере понадобится наличие диплома о высшем образовании. Однако очень часто в жизни случается так, что сложные обстоятельства мешают успешно окончить учебу, получая такой важный документ.
Приобрести диплом университета
Мы предлагаем быстро и выгодно заказать диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями. Диплом способен пройти любые проверки, даже с использованием специально предназначенного оборудования. Достигайте цели быстро и просто с нашим сервисом.
Где приобрести диплом специалиста? tutdiploms.com/
Mazrueo
| #
Мы изготавливаем дипломы любой профессии по невысоким ценам. Стараемся поддерживать для клиентов адекватную ценовую политику. Для нас важно, чтобы документы были доступными для большого количества граждан.
Покупка диплома, который подтверждает обучение в университете, – это выгодное решение. Заказать диплом любого ВУЗа: kofe.80lvl.ru/posting.php?mode=post&f=10&sid=cac5f4df6dd5083689cc7ee1b3b23500
Sazrros
| #
спб купить диплом
Lazrfso
| #
Всё, что нужно знать о покупке аттестата о среднем образовании без рисков
Xazrodx
| #
Можно ли купить аттестат о среднем образовании? Основные рекомендации
online casino lDic
| #
You actually said this terrifically.
tropicana online casino reviews https://casinoslotssaid.com/online-casino-georgia/ book of shadows online casino
JesseEvedy
| #
https://london-best.com/
RonaldToulp
| #
Профессиональное производство светодиодных экранов здесь, узнайте о наших услугах, смотрите тут https://conifer.rhizome.org/LedMedia/
Diplomi_nlSa
| #
где купить диплом техникума где купить диплом техникума .
Diplomi_ujKi
| #
купить диплом по физической культуре 2orik-diploms.ru .
online casino lDic
| #
You mentioned this fantastically!
como abrir un casino online https://linkscasino.info/golf-betting/ genting casino online phone number
Cazrqcg
| #
Заказать диплом ВУЗа поспособствуем- diplomybox.com/kupit-diplom-tekhnikuma-kolledzha-v-sankt-peterburge
Huaweicaree
| #
Ремонт смартфонов Huawei — популярная услуга, поскольку даже самые надёжные устройства могут выйти из строя. Разберёмся в распространённых поломках, их причинах и примерной стоимости ремонта.
здесь
Cazrkmg
| #
Мы изготавливаем дипломы любых профессий по приятным ценам. — flashboot.ru/profile/diplomygroup
Diplomi_wjSr
| #
купить диплом вуза в красноярске diploms-bests.ru .
ScottGot
| #
аренда теплоходов цены сколько стоит прокат яхт
RonaldZitle
| #
яхта в дубай яхта на 12 человек
Altonamoup
| #
мтс кемерово
https://29sixservices.in/employer/will/
мтс тв
online casino lDic
| #
Wonderful knowledge. Thanks a lot.
new casino online real money usa https://buckscasino.info/review-xbet/ casino spiele kostenlos online
online casino lDic
| #
Nicely put, Regards!
best online bonus casino https://findscasino.info/horse-betting/ casino online bonus free spins
online casino lDic
| #
Seriously lots of wonderful tips!
best arizona online casino https://casinosonlinenew.com/online-casino-virginia/ g online casino
online casino lDic
| #
Superb forum posts. Thank you.
fun city casino online https://hotgamblingguide.info/new-jersey-online-casino-websites/ mrq online casino
Huaweicaree
| #
Ремонт смартфонов Huawei — популярная услуга, поскольку даже самые надёжные устройства могут выйти из строя. Разберёмся в распространённых поломках, их причинах и примерной стоимости ремонта.
перейти
Petersig
| #
мтс цены
https://ambitech.com.br/employer/timmy/
мтс интернет
online casino lDic
| #
Thanks a lot! Very good information.
online casino im test https://onlinecasinoindex.us/online-casino-baccarat/ trusted usa online casinos
online casino lDic
| #
You’ve made your position pretty clearly.!
the river casino online https://casinoshaman.com/maryland-live-casino-online-gambling/ vilket online casino är bäst
online casino lDic
| #
Regards. Plenty of content.
zone online casino login https://combatcasino.info/mbl-betting/ online casino free 50
online casino lDic
| #
With thanks! Fantastic information!
sweepstakes online casinos https://usagamblingexperts.com/sports-betting-sites/ como cobrar casino online
JamesTom
| #
мегафон домашний интернет ростов
https://www.lotusprotechnologies.com/companies/mallory/
мегафон цены
online casino lDic
| #
You actually said it really well!
paypal and online casinos https://casinoslotoking.com/best-online-casino-north-carolina/ ocean ac online casino
online casino lDic
| #
Incredible all kinds of terrific material!
bonus code for resorts casino online https://cryptogamblingguru.com/bet-on-tennis/ ph bet online casino
JesseEvedy
| #
https://london-best.com/
online casino lDic
| #
You expressed this perfectly.
jackpotcity online casino https://casinocashstars.com/review-ducky-luck/ casino training online
online casino lDic
| #
Thanks. Quite a lot of advice!
comprar software de casino online argentina https://shadowcasino.info/real-money-online-casino-georgia/ nv casino online
Williamhab
| #
https://luxembourg-best.com/
BryanOxisk
| #
провайдер ттк
https://hitechjobs.me/companies/ttk-tarif-llc/
ттк тв
Williamhab
| #
https://luxembourg-best.com/
programmi 1s _oxsa
| #
1 с программа 1 с программа .
programmi 1s _bfSa
| #
программа 1с бухгалтерия купить в москве программа 1с бухгалтерия купить в москве .
silovie transformatori kypit_cgoa
| #
силовой трансформатор цена силовой трансформатор цена .
online casino lDic
| #
Regards. An abundance of content!
online casino no limit https://riggambling.com/golf-betting/ sugarhouse casino online promo code
грузовые шины от производителя оптом
| #
Aplus11 – оптимальное решение для грузовых машин.
грузовые шины от производителя оптом
online casino lDic
| #
Nicely put, Cheers!
free online casinos no download no registration https://magicalcasino.info/review-xbet/ online casino fair play
online casino lDic
| #
Kudos, An abundance of material.
cleopatra slot online casino https://riggambling.com/online-casino-canada/ situs casino online terbaik
грузовые шины от производителя оптом
| #
DOUBLECOIN11 – качество, проверенное годами.
грузовые шины от производителя оптом
online casino lDic
| #
Thanks. I enjoy it.
casino royale movie free online https://igamingcasino.info/review-busr/ red cherry online casino
LelandKarve
| #
ттк подключение ростов
https://www.mafiscotek.com/employer/owen/
ттк тв ростов
online casino lDic
| #
Regards, Ample content.
complete list of nj online casinos https://eseomail.com/banking/ netbet casino online
slot777
| #
i will never forget about this, this help me a lot ser, i really appreciate everything youve done ser, thanks ser
Inscreva-se para receber 100 USDT
| #
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
DonaldPhivy
| #
билайн домашний интернет кемерово
https://getquikjob.com/employer/gennie/
билайн подключить
Aaronfoets
| #
macbook air pro m2 macbook air m2 15
RichardReumb
| #
ноутбук apple macbook pro 16 1tb https://apple-macbook-pro213.ru
Kxyufaita
| #
darknet websites bitcoin dark web
WilliamIRrutty
| #
dark market url https://github.com/newonionlinks/darknetmarkets – dark markets 2025
MarkNOlaf
| #
darknet drug store https://github.com/newonionlinks/darknetmarkets – darknet websites
FNDaviditabe
| #
darkmarket link https://github.com/newonionlinks/darknetmarkets dark web markets
online casino lDic
| #
Great material, Appreciate it!
sc casinos online no deposit bonus https://hotgamblingguide.com/omaha-online-poker/ reddit best online casino
Altonamoup
| #
мтс тарифы на интернет
https://www.homebasework.net/employer/winifred/
провайдер мтс
Aaronfoets
| #
macbook air 13 m2 https://macbook-air-m2.ru
RichardReumb
| #
apple macbook pro 14 m4 apple macbook pro 16 max
online casino lDic
| #
Good stuff. Thanks a lot!
barcrest online casinos https://casinoslotoking.com/real-online-poker-for-real-money/ agent ligaz888 casino online
Kxyufaita
| #
darkmarket dark web sites
WilliamIRrutty
| #
dark web sites https://github.com/newonionlinks/darknetmarkets – dark market
MarkNOlaf
| #
dark market link https://github.com/newonionlinks/darknetmarkets – darknet websites
online casino lDic
| #
You expressed it exceptionally well.
winward casino online https://findscasino.info/omaha-poker-online/ ezugi casino online
FNDaviditabe
| #
darknet drug store https://github.com/newonionlinks/darknetmarkets dark web market list
silovie transformatori kypit_kckl
| #
высоковольтный трансформатор купить высоковольтный трансформатор купить .
top-mmorpg
| #
Поделился с друзьями надежным букмекером – букмекерская контора 1 win. Все оценили скорость доступа и стабильность работы. Больше никаких срывов ставок из-за блокировок или технических проблем.
RickeyExofe
| #
weekend yacht rentals yacht birthday party
Petersig
| #
провайдер мтс
https://pivotalta.com/employer/1771/bert/
мтс подключить краснодар
online casino lDic
| #
You actually said this wonderfully.
holland casino online spelen https://combatcasino.info/real-money-online-casino-texas/ astropay card casinos online
Michaelbof
| #
кракен ссылка онлайн kra30.at
Kxyufaita
| #
dark web market darknet websites
WilliamIRrutty
| #
dark market url https://github.com/newonionlinks/darknetmarkets – dark web market list
MarkNOlaf
| #
dark web markets https://github.com/newonionlinks/darknetmarkets – dark web sites
top-mmorpg
| #
Официальный ресурс 1xbet официальный сайт гарантирует безопасность ваших данных и честную игру. Современный интерфейс, удобная навигация и быстрые транзакции – все для вашего комфорта.
FNDaviditabe
| #
dark web market urls https://github.com/newonionlinks/darknetmarkets darkmarket url
online casino lDic
| #
Thank you. Quite a lot of advice.
best online blackjack casino reddit https://casinoslotoking.com/crypto-casinos-online/ welcome bonus online casino
mostbet_rnka
| #
mostbet uzbekistan mostbet3020.ru .
mostbet_ebka
| #
1 вин скачать https://1win36.com.kg/ .
Kxyufaita
| #
darknet market list darknet markets url
online casino lDic
| #
Fantastic information, Cheers!
legit slot online casino https://hotgamblingguide.info/table-tennis-betting/ coushatta casino online
MarkNOlaf
| #
dark market https://github.com/newonionlinks/darknetmarkets – best darknet markets
WilliamIRrutty
| #
darknet sites https://github.com/newonionlinks/darknetmarkets – darknet sites
FNDaviditabe
| #
darknet market list https://github.com/newonionlinks/darknetmarkets darknet market list
online casino lDic
| #
Nicely put. Kudos.
www royal casino online com https://igamingcasino.info/review-slotocash/ online casino south africa legal
mostbet_heEl
| #
masbet masbet .
iPadcaree
| #
Проблемы с зарядкой iPad могут возникнуть неожиданно и доставить массу неудобств. Причины таких проблем разнообразны — от тривиальных до серьёзных. Прежде чем обращаться в сервисный центр, можно попробовать решить проблему самостоятельно. Эта статья поможет вам разобраться в основных причинах и способах их устранения.
подробнее
JamesTom
| #
мегафон подключение ростов
https://cvbankye.com/employer/damon/
мегафон интернет ростов
Kxyufaita
| #
darknet market darknet market
MarkNOlaf
| #
darkmarket 2025 https://github.com/newonionlinks/darknetmarkets – dark web market links
WilliamIRrutty
| #
darknet drugs https://github.com/newonionlinks/darknetmarkets – darkmarket
Michaelbof
| #
кракен актуальная ссылка на сегодня kra at
Kevinfieby
| #
book a private yacht tour dubai boat
FNDaviditabe
| #
onion dark website https://github.com/newonionlinks/darknetmarkets darkmarket list
online casino lDic
| #
Regards! Good information!
online casino gambling in michigan https://onlinecasinoindex.us/new-zealand-online-casino/ gta online diamond casino
online casino lDic
| #
Nicely put. Thank you!
5$ deposit online casinos https://casinocashstars.com/sportsbooks/ happy game online casino download
mostbet_qxPn
| #
мостбет войти http://mostbet3019.ru .
JerryGlymn
| #
https://t.me/ASOIMMADV
Kxyufaita
| #
darknet drug links darkmarket 2025
MarkNOlaf
| #
dark market url https://github.com/newonionlinks/darknetmarkets – dark web markets
online casino lDic
| #
Awesome material. With thanks.
minimum deposit online casino https://mgmonlinecasino.us/tennessee-online-casinos/ table casino games online
BryanOxisk
| #
сайт ттк барнаул
https://neuronerecruitment.com.au/employer/ttk-tarif-solutions/
ттк телевидение
Sazrupv
| #
Здравствуйте!
Заказать диплом ВУЗа по доступной стоимости возможно, обращаясь к проверенной специализированной компании. Заказать диплом о высшем образовании: good-diplom.ru/kupit-diplom-chelyabinsk/
online casino lDic
| #
Nicely put, Thanks a lot!
sisal casino online https://casinosonlinenew.com/ oasis online casino
Aaronnut
| #
кракен зеркало 2025 кракен купить
MichaelKelay
| #
The best HD wallpapers https://wallpapers-all.com/62299-celebrity-chloe-grace-moretz-wallpaper.html in one place! Download free backgrounds for your desktop and smartphone. A huge selection of pictures – from minimalism to bright landscapes and fantasy. Enjoy stylish images every day!
Terencesam
| #
фриспины без депозита с выводом за регистрацию бонусы без отыгрыша за регистрацию
Pingrutty
| #
darknet markets url dark markets 2025
DonDonfaita
| #
dark market 2025 darknet market list
Donaldlaf
| #
dark web market dark markets
Toliknaf
| #
dark web markets darknet drugs
Rabyitabe
| #
darkmarkets darknet site
Samsungcaree
| #
Если на вашем телефоне Samsung Galaxy экран некорректно реагирует на прикосновения или не работает вовсе, не спешите паниковать. Сейчас мы рассмотрим основные шаги, которые помогут решить проблему. И на примере Samsung S20/21/22/23/24 пошагово выполним все действия, которые могут восстановить работоспособность экрана.
перейти
Kxyufaita
| #
dark markets darknet drug links
WilliamIRrutty
| #
dark market list https://github.com/newonionlinks/darknetmarkets – darknet markets
online casino lDic
| #
Thank you, Quite a lot of forum posts.
casino on gta online https://onlinecasinoindex.us/mybookie-sign-up-bonus/ band new real cas winnings online casinos 2019
Cazrpqd
| #
Мы готовы предложить дипломы любой профессии по выгодным тарифам. Купить диплом Новочеркасск — kyc-diplom.com/geography/novocherkassk.html
Cazrchf
| #
Приобрести диплом любого ВУЗа поспособствуем. Купить диплом магистра в Томске – diplomybox.com/kupit-diplom-magistra-v-tomske
online casino lDic
| #
Well expressed indeed! !
victory online casino https://onlinecasinoindex.us/casino-online-california/ mohegan sun online casino bonus code
LelandKarve
| #
ттк тарифы ростов
https://jobsleed.com/companies/chandra/
ттк подключение
Pingrutty
| #
dark market link dark market onion
DonDonfaita
| #
darknet market darknet links
Toliknaf
| #
dark web sites darknet market links
Rabyitabe
| #
darknet sites darknet drug market
Kxyufaita
| #
darknet markets onion address dark web market links
MarkNOlaf
| #
darknet market links https://github.com/newonionlinks/darknetmarkets – darknet markets url
online casino lDic
| #
Thanks a lot, An abundance of advice!
lucky creek online casino review https://hotgamblingguide.info/minnesota-casinos-online/ usa online casino no deposit bonus keep what you win
online casino lDic
| #
Information clearly utilized.!
online casino med dansk licens https://casinosonlinenew.com/review-cafe/ free no deposit online casino codes
Pingrutty
| #
darknet markets links tor drug market
1win_erkn
| #
1win casino en línea 1win2.com.mx .
1win_vget
| #
1 вин скачать 1 вин скачать .
Aaronnut
| #
ссылка кракен сайт зеркало kra29. cc
MichaelKelay
| #
The best HD wallpapers https://wallpapers-all.com/4384-lego-hero-factory.html in one place! Download free backgrounds for your desktop and smartphone. A huge selection of pictures – from minimalism to bright landscapes and fantasy. Enjoy stylish images every day!
Donaldlaf
| #
darknet drugs darknet websites
Toliknaf
| #
darknet markets onion address dark web markets
Kxyufaita
| #
dark market onion darknet markets 2025
WilliamIRrutty
| #
darkmarket url https://github.com/newonionlinks/darknetmarkets – dark market 2025
MarkNOlaf
| #
dark markets 2025 https://github.com/newonionlinks/darknetmarkets – darknet marketplace
online casino lDic
| #
Many thanks! An abundance of knowledge!
mohegan sun online casino pa promo code no deposit https://hotgamblingguide.com/shazam-casino-bonus-codes/ online casino real money michigan
Pingrutty
| #
dark web markets darknet marketplace
online casino lDic
| #
Nicely put. Thank you.
online casino topless dealer https://riggambling.com/online-keno/ vincere casino online
Toliknaf
| #
dark market url dark markets 2025
Terencesam
| #
бездепозитные игровые автоматы Бездепозитные бонусы за регистрацию
Donaldlaf
| #
dark web drug marketplace dark web marketplaces
WilliamIRrutty
| #
dark web marketplaces https://github.com/newonionlinks/darknetmarkets – darknet websites
Kxyufaita
| #
dark market darkmarket link
Rabyitabe
| #
dark market dark market link
silovie transformatori kypit_ssei
| #
купить трансформатор силовой https://www.silovye-transformatory-kupit11.ru .
online casino lDic
| #
Seriously lots of wonderful knowledge!
jubilee casino online https://casinoslotssaid.com/betting/ fortune coin online casino
Pingrutty
| #
dark web link dark market link
programmi 1s_rwSn
| #
купить 1с в москве купить 1с в москве .
1win_uxot
| #
1вин онлайн http://www.1win41.com.kg .
mostbet_sser
| #
мостбет скачать приложение на андроид мостбет скачать приложение на андроид .
online casino lDic
| #
Wonderful forum posts. Many thanks.
online slots casino real money https://combatcasino.info/online-casino-washington/ banger casino online
MarkNOlaf
| #
dark market url https://github.com/newonionlinks/darknetmarkets – dark market url
WilliamIRrutty
| #
darknet markets links https://github.com/newonionlinks/darknetmarkets – darknet markets 2025
Toliknaf
| #
dark web market urls darknet markets
Pingrutty
| #
onion dark website dark web sites
Kxyufaita
| #
dark markets 2025 dark web market
Donaldlaf
| #
dark market dark web markets
online casino lDic
| #
Very good write ups. Regards!
online casino with trustly https://hotgamblingguide.com/betting-in-boxing/ casino online ruleta el salvador
Rabyitabe
| #
dark markets 2025 dark web markets
JerryGlymn
| #
defi news crypto regulations
online casino lDic
| #
Many thanks! I like it!
mississippi online casinos https://hotgamblingguide.com/best-online-casino-north-carolina/ free online casino games with no download or registration
RonaldKinge
| #
монтаж системы вентиляции в частном доме
Pingrutty
| #
darknet sites dark web drug marketplace
WilliamIRrutty
| #
darknet drug store https://github.com/newonionlinks/darknetmarkets – dark websites
Kxyufaita
| #
dark web link dark markets 2025
online casino lDic
| #
Awesome stuff. Appreciate it.
what online casino does drake play https://casinoslotssaid.com/betting-sites-cricket/ new casino online games
Toliknaf
| #
darknet markets darknet market list
NathanAsymn
| #
Страхование по лучшей цене https://осагополис.рф Сравните предложения страховых компаний и выберите полис с выгодными условиями. Удобный сервис поможет найти оптимальный вариант автострахования, ОСАГО, КАСКО, туристических и медицинских страховок.
Donaldlaf
| #
dark web sites tor drug market
KennethTalry
| #
займ онлайн бесплатно займ сайт
Rabyitabe
| #
darknet markets dark market link
online casino lDic
| #
You’ve made your point pretty clearly!!
new online casinos nj https://hotgamblingguide.info/poker-game-online/ sloty online casino
NathanAsymn
| #
Страхование по лучшей цене https://осагополис.рф Сравните предложения страховых компаний и выберите полис с выгодными условиями. Удобный сервис поможет найти оптимальный вариант автострахования, ОСАГО, КАСКО, туристических и медицинских страховок.
KennethTalry
| #
займ онлайн сайт займ онлайн
Pingrutty
| #
dark web markets darknet markets onion
WilliamIRrutty
| #
dark web drug marketplace https://github.com/newonionlinks/darknetmarkets – dark web market links
MarkNOlaf
| #
darknet drug market https://github.com/newonionlinks/darknetmarkets – darknet links
Kxyufaita
| #
dark web market urls dark web marketplaces
online casino lDic
| #
Regards, An abundance of tips.
online sweeps casino https://onlinecasinoindex.us/nhl-hockey-betting/ bay mills resort & casino online casinos michigan
DonDonfaita
| #
onion dark website darknet markets onion
Donaldlaf
| #
darknet market links dark market list
RichardRhymn
| #
Ремонт кофемашин в Москве https://coffee-help24.ru быстро, качественно, с гарантией! Обслуживаем все бренды: Saeco, DeLonghi, Jura, Bosch и др. Диагностика, замена деталей, чистка от накипи. Выезд мастера на дом или ремонт в сервисе.
online casino lDic
| #
Wow plenty of valuable advice!
juegos de casino las vegas online gratis https://combatcasino.info/real-money-online-casino-minnesota/ four winds casino online gaming
FNDaviditabe
| #
bitcoin dark web https://github.com/newonionlinks/darknetmarkets darknet markets 2025
Terencesam
| #
бонусы за регистрацию 1xslots бездепозитный бонус
Pingrutty
| #
dark web marketplaces dark web market
RichardRhymn
| #
Ремонт кофемашин в Москве https://coffee-help24.ru быстро, качественно, с гарантией! Обслуживаем все бренды: Saeco, DeLonghi, Jura, Bosch и др. Диагностика, замена деталей, чистка от накипи. Выезд мастера на дом или ремонт в сервисе.
RobertSox
| #
Микрозайм взять – займы интернет
online casino lDic
| #
Thanks. I like it!
online gaming casino real money https://casinoslotoking.com/play-texas-holdem-online-for-real-money/ all slots casino online chat
Allandoums
| #
Последние IT-новости https://notid.ru быстро и понятно! Рассказываем о цифровых трендах, инновациях, стартапах и гаджетах. Только проверенная информация, актуальные события и мнения экспертов. Оставайтесь в центре IT-мира вместе с нами!
MarkNOlaf
| #
darknet site https://github.com/newonionlinks/darknetmarkets – dark web marketplaces
WilliamIRrutty
| #
dark markets https://github.com/newonionlinks/darknetmarkets – bitcoin dark web
online casino lDic
| #
Valuable write ups. Thank you!
best canadian online casino payouts https://casinonair.com/online-casino-georgia/ gta online casino heist hacking device location
Toliknaf
| #
dark web link darknet drug links
Rabyitabe
| #
darknet markets dark market url
Kxyufaita
| #
darknet markets links darknet websites
FNDaviditabe
| #
darknet marketplace https://github.com/newonionlinks/darknetmarkets darknet market lists
Donaldlaf
| #
darknet market darknet markets onion address
Allandoums
| #
Последние IT-новости https://notid.ru быстро и понятно! Рассказываем о цифровых трендах, инновациях, стартапах и гаджетах. Только проверенная информация, актуальные события и мнения экспертов. Оставайтесь в центре IT-мира вместе с нами!
Pingrutty
| #
darkmarket darknet markets url
RobertPsype
| #
yacht trip dubai private yacht
online casino lDic
| #
You actually suggested it terrifically.
casino games online india https://mapcasino.info/review-ignition/ casino de montreal online
MarkNOlaf
| #
dark web sites https://github.com/newonionlinks/darknetmarkets – darknet markets 2025
WilliamIRrutty
| #
darknet drug market https://github.com/newonionlinks/darknetmarkets – darknet sites
online casino lDic
| #
Fantastic content. Kudos.
mwplay888 online casino login https://riggambling.com/nascar-betting/ 10$ deposit online casino
RonaldKinge
| #
оформление дендроплана
DonDonfaita
| #
bitcoin dark web darknet markets onion address
Toliknaf
| #
dark web market dark web markets
Kxyufaita
| #
darkmarket url dark market link
FNDaviditabe
| #
darknet marketplace https://github.com/newonionlinks/darknetmarkets darknet drug links
Rabyitabe
| #
onion dark website darknet site
Pingrutty
| #
darkmarket 2025 darknet markets 2025
Donaldlaf
| #
darknet market list dark websites
Samsungcaree
| #
Современные телевизоры Samsung, такие как QLED QN90C, OLED S95B и Neo QLED 8K QN800A, полны передовых функций — от невероятного качества изображения до возможностей Smart TV. Однако даже у этих флагманских моделей могут возникать проблемы:
подробнее
mostbet_veOa
| #
авиатор игра на деньги http://mostbet11.com.kg .
mostbet_gxMl
| #
mostbet.con http://mostbet12.com.kg .
MarkNOlaf
| #
darknet marketplace https://github.com/newonionlinks/darknetmarkets – darknet markets url
online casino lDic
| #
Amazing posts. Regards.
star casino online game https://mapcasino.info/new-online-casinos/ twin rivers casino online
Pingrutty
| #
dark market darknet market lists
Kxyufaita
| #
darkmarket url darkmarkets
FNDaviditabe
| #
darkmarket link https://github.com/newonionlinks/darknetmarkets darknet market
DonDonfaita
| #
dark markets darknet markets
Toliknaf
| #
darknet markets onion dark market 2025
RadikCype
| #
https://provocation-tattoo.ru/news/pgs/?ozghidaemue_filmu_1.html что посмотреть из фильмов интересного на вечер
Rabyitabe
| #
dark market 2025 dark websites
Donaldlaf
| #
bitcoin dark web bitcoin dark web
online casino lDic
| #
Nicely put, Appreciate it!
online casino target audience https://combatcasino.info/fast-payout-casino/ maine online casino
Pingrutty
| #
darkmarket dark websites
WilliamIRrutty
| #
darknet market links https://github.com/newonionlinks/darknetmarkets – darknet links
Kxyufaita
| #
dark websites darknet market links
FNDaviditabe
| #
darknet market https://github.com/newonionlinks/darknetmarkets darknet market list
MatthewbiB
| #
букет цветов купить спб с доставкой букет цветов заказать с доставкой
Toliknaf
| #
dark market url dark web market
Samsungcaree
| #
Современные телевизоры Samsung, такие как QLED QN90C, OLED S95B и Neo QLED 8K QN800A, полны передовых функций — от невероятного качества изображения до возможностей Smart TV. Однако даже у этих флагманских моделей могут возникать проблемы:
здесь
online casino lDic
| #
Superb data. Cheers!
online casino games using paypal https://uscasinoguides.com/maryland-casinos/ planet casino online
Rabyitabe
| #
dark web link darknet market
Donaldlaf
| #
darknet drug links darknet markets
online casino lDic
| #
You said it perfectly.!
pa live casino online https://casinonair.com/tennis-betting/ real online casino with no deposit bonus
MatthewbiB
| #
доставка букетов цветов цветы питер купить
Pingrutty
| #
darknet market lists darknet websites
binance
| #
Thanks for sharing. I read many of your blog posts, cool, your blog is very good. https://accounts.binance.com/pt-BR/register-person?ref=YY80CKRN
MarkNOlaf
| #
darknet marketplace https://github.com/newonionlinks/darknetmarkets – darknet drug links
RadikCype
| #
https://evu.uz/wp-content/pgs/sovetskoe_kino__glavnoe.html бандитский петербург песня корнелюка слушать бесплатно
FNDaviditabe
| #
darknet sites https://github.com/newonionlinks/darknetmarkets darknet market links
DonDonfaita
| #
dark markets dark market 2025
online casino lDic
| #
You actually mentioned that terrifically.
online live casino reviews https://shadowcasino.info/online-casino-colorado/ texas online casino
Rabyitabe
| #
darknet markets onion address darknet site
Donaldlaf
| #
dark web drug marketplace darknet websites
online casino lDic
| #
Regards. I appreciate it!
online casino exchange https://shadowcasino.info/live-online-casinos/ ten online casino
Pingrutty
| #
dark web drug marketplace dark web sites
phoenixautobodyshopmailm
| #
Unlock your vehicle’s potential with our top-notch auto tuning services! Unleash your ride into a stunning masterpiece with our expert team. We specialize in refinements that cater to your unique style. Our reliable solutions ensure optimal performance and aesthetic appeal . Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://phoenix-autobodyshop.com/ today and take the first step towards your dream car!
Samsungcaree
| #
Как транслировать содержимое с телефона Samsung на телевизор через WI-FI или DeX кабель. Пошаговая инструкция всех вариантов
ссылка
MarkNOlaf
| #
darkmarket 2025 https://github.com/newonionlinks/darknetmarkets – dark market list
WilliamIRrutty
| #
darknet market lists https://github.com/newonionlinks/darknetmarkets – darknet drugs
Terencesam
| #
бонусы за регистрацию фриспины без депозита за регистрацию с выводом
Kxyufaita
| #
darkmarket url darkmarket url
online casino lDic
| #
Cheers! I like this.
bob online casino https://eseomail.com/texas-holdem-online/ casino canada online
Toliknaf
| #
dark web link darknet markets
Curtiswoums
| #
кракен вход ссылка kraken зайти без впн
Pingrutty
| #
best darknet markets dark markets 2025
Rabyitabe
| #
dark web market best darknet markets
online casino lDic
| #
Point well utilized!!
1 euro deposit casino online https://usagamblingexperts.com/online-slots/ jupiters casino online
Donaldlaf
| #
onion dark website darknet markets url
WilliamIRrutty
| #
dark market 2025 https://github.com/newonionlinks/darknetmarkets – dark market link
Sazrpza
| #
начальный аттестат купить
Kxyufaita
| #
darknet market list dark market
TCLcaree
| #
Телевизоры TCL с функцией Smart TV предоставляют доступ к потоковым видеосервисам, приложениям и другим онлайн-возможностям. Однако некоторые пользователи могут столкнуться с проблемой подключения к Wi-Fi. Давайте разберем основные причины этой неисправности и предложим эффективные методы ее устранения.
ссылка
Dnrtylu
| #
реально купить диплом о среднем специальном образовании
online casino lDic
| #
Really all kinds of great material!
online casino virtual money https://combatcasino.info/review-cafe/ jeetwin online casino bd
DonDonfaita
| #
darknet marketplace onion dark website
Pingrutty
| #
darknet markets url darknet market links
online casino lDic
| #
Nicely expressed really! !
open online casino https://snipercasino.info/online-video-poker/ casino nederland online
Rabyitabe
| #
darknet drugs darknet drug market
WilliamIRrutty
| #
darknet drug store https://github.com/newonionlinks/darknetmarkets – dark markets
Donaldlaf
| #
dark web drug marketplace dark web markets
MarkNOlaf
| #
darknet links https://github.com/newonionlinks/darknetmarkets – dark market url
Robertflags
| #
производство мебели Шкаф-купе на заказ от производителя – это не просто предмет мебели, а инвестиция в комфорт и функциональность вашего дома. Индивидуальный подход позволяет максимально эффективно использовать пространство, учитывая особенности планировки и ваши личные предпочтения в дизайне. Вы получаете возможность выбрать материалы, цвет, внутреннее наполнение и размеры, создавая шкаф, идеально вписывающийся в интерьер и отвечающий всем вашим потребностям.
Kxyufaita
| #
darknet marketplace dark markets 2025
Pingrutty
| #
dark web marketplaces dark markets
online casino lDic
| #
Appreciate it! Lots of material.
casinos slots online https://findscasino.info/online-keno/ lucky penny casino online
Toliknaf
| #
darknet drug store darkmarket list
DonDonfaita
| #
darknet market lists darkmarkets
online casino lDic
| #
Thank you, Good information!
offshore online casinos https://casinoslotoking.com/countries/ online casinos indiana
WilliamIRrutty
| #
dark web link https://github.com/newonionlinks/darknetmarkets – darknet market links
MarkNOlaf
| #
darkmarket link https://github.com/newonionlinks/darknetmarkets – dark web market list
Rabyitabe
| #
darknet market list darknet websites
Donaldlaf
| #
dark web market darknet markets onion address
phoenixautobodyshopmailm
| #
Unlock your vehicle’s potential with our top-notch auto tuning services! Discover your ride into a stunning masterpiece with our expert team. We specialize in upgrades that cater to your unique style. Our exceptional solutions ensure optimal performance and stylish look . Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://phoenix-autobodyshop.com/ today and take the first step towards your dream car!
Kxyufaita
| #
darknet markets url darknet drug links
Pingrutty
| #
onion dark website bitcoin dark web
online casino lDic
| #
Nicely put. Thanks.
australian online casino reviews 2018 https://magicalcasino.info/virginia-online-casinos/ online casino 10 euro startguthaben
mostbet_fbsl
| #
мост бет официальный сайт http://www.mostbet13.com.kg/ .
mostbet_rhmn
| #
состбет состбет .
DonDonfaita
| #
dark markets darkmarket list
Toliknaf
| #
darknet market links dark market link
online casino lDic
| #
You said it nicely..
mgm online casino gift card https://mgmonlinecasino.us/casino-wild/ free online virtual casino
WilliamIRrutty
| #
darknet drugs https://github.com/newonionlinks/darknetmarkets – darknet links
Terencesam
| #
свежие фриспины риобет какие автоматы дают деньги за регистрацию без депозита
Rabyitabe
| #
darknet markets onion dark web market
Kxyufaita
| #
darknet links darknet markets url
Donaldlaf
| #
darknet market list dark market 2025
Pingrutty
| #
darknet market lists darknet drug links
online casino lDic
| #
Nicely put. Cheers.
casino venezia online https://igamingcasino.info/horse-betting/ best payout online casinos usa
MarkNOlaf
| #
darknet market https://github.com/newonionlinks/darknetmarkets – darknet drug links
Toliknaf
| #
dark markets 2025 darknet websites
online casino lDic
| #
Nicely put. Thanks.
gta online casino work missions https://hotgamblingguide.com/golf-betting-online/ slot games online casino
RadikCype
| #
http://interra.com.ru/media/pgs/?filmu_onlayn_v_horoshem_kachestve.html белый ангел ростов на дону снять
Kxyufaita
| #
darkmarket link dark markets
Pingrutty
| #
bitcoin dark web darknet markets onion address
Rabyitabe
| #
darknet drugs darkmarkets
Donaldlaf
| #
darknet drugs darknet market list
WilliamIRrutty
| #
darkmarket https://github.com/newonionlinks/darknetmarkets – dark web drug marketplace
MarkNOlaf
| #
dark web sites https://github.com/newonionlinks/darknetmarkets – dark web market list
online casino lDic
| #
Beneficial knowledge. Cheers!
online casinos with freeplay https://mgmonlinecasino.us/bingo-online-for-money/ ocean online casino download
DonDonfaita
| #
darknet drugs darknet markets onion
Toliknaf
| #
dark market link darkmarket url
Pingrutty
| #
darknet markets links darknet markets onion
online casino lDic
| #
Truly lots of valuable tips!
online casino promotions no deposit bonus https://usagamblingexperts.com/real-money-roulette/ best online casino in america
Kxyufaita
| #
darknet market list dark web markets
Trefgxg
| #
Заказать документ о получении высшего образования можно в нашей компании. dip-lom-rus.ru/kupit-diplom-lipetsk
Jariorrzi
| #
Привет!
Начальники очень часто выбирают претендентов, которые закончили ВУЗ. Особенно ценятся элитные учебные заведения. Впрочем учиться 5 лет – это долго и дорого, далеко не у всех есть подобная возможность. Заказать документ становится самым оптимальным решением.
Бывают и непредвиденные случаи, когда диплом теряется или портится. Далеко не всегда возможно быстро и без проблем восстановить его, особенно если ВУЗ закрыт или располагается где-то в другом регионе страны. Бюрократия отнимает множество времени.
Для быстрого продвижения по карьере понадобится наличие диплома института. Но очень часто в жизни может случиться так, что сложные обстоятельства не дают с успехом окончить учебу и заполучить такой важный документ.
Купить диплом университета
Мы предлагаем выгодно и быстро приобрести диплом, который выполнен на оригинальной бумаге и заверен печатями, водяными знаками, подписями официальных лиц. Наш диплом способен пройти любые проверки, даже при помощи специальных приборов. Решайте свои задачи максимально быстро с нашими дипломами.
Где заказать диплом по необходимой специальности? tutdiploms.com/
Rabyitabe
| #
darknet markets darkmarket list
RadikCype
| #
https://lorrd.ru/test/pgs/doramu_na_kinogo___mir_vostochnoy_dramu_i_yarkih_istoriy.html м видео белая дача телефон
Donaldlaf
| #
darknet markets onion address dark web market urls
mostbet_tjOn
| #
мотбет mostbet19.com.kg .
Diplomi_zuol
| #
купить диплом мед образования купить диплом мед образования .
WilliamIRrutty
| #
darknet sites https://github.com/newonionlinks/darknetmarkets – dark market url
1win_weka
| #
1вин вход 1вин вход .
MarkNOlaf
| #
darknet drug links https://github.com/newonionlinks/darknetmarkets – dark web market urls
online casino lDic
| #
Appreciate it. Lots of advice!
bandar betting casino 368bet online https://ratingcasino.info/horse-betting/ isle of man casino online
Pingrutty
| #
darkmarket list dark markets 2025
Kxyufaita
| #
dark web market links darkmarket link
online casino lDic
| #
Many thanks! I value it!
paf online casino https://uscasinoguides.com/massachusetts-casinos/ gta online casino heist silent and sneaky best approach
Curtiswoums
| #
кракен маркетплейс ссылка kraken вход
FNDaviditabe
| #
darknet market list https://github.com/newonionlinks/darknetmarkets dark web sites
WilliamIRrutty
| #
darknet markets url https://github.com/newonionlinks/darknetmarkets – dark web marketplaces
online casino lDic
| #
Useful knowledge. Many thanks.
best payout usa online casino https://usagamblingexperts.com/real-money-blackjack/ euro grand online casino
Toliknaf
| #
darknet markets onion address darkmarket url
online casino lDic
| #
Many thanks. Very good stuff.
australian online casino no deposit https://casinoslotssaid.com/best-sportsbook-bonuses/ online casino tropez
Rabyitabe
| #
darknet market links tor drug market
Donaldlaf
| #
dark market link onion dark website
mostbet_oqpl
| #
mostbet промокод https://www.mostbet20.com.kg .
Epsoncaree
| #
Настройка Wi-Fi на принтерах Epson, таких, как модели L3250 и L3251, позволяет значительно упростить процесс печати и сканирования. Вы сможете печатать документы прямо с компьютера, смартфона или планшета, не используя провода.
подробнее
Terencesam
| #
Бонусы за регистрацию Игровые автоматы на деньги играть
Pingrutty
| #
darknet drugs dark websites
Toliknaf
| #
tor drug market onion dark website
online casino lDic
| #
Thank you. A good amount of facts.
bestes zimpler online casino https://onlinecasinoindex.us/australian-online-casino-no-deposit-bonus/ philippines online casino pagcor
DonDonfaita
| #
darknet site dark market 2025
online casino lDic
| #
Really a lot of valuable tips.
is vegas online casino legit https://casinonair.com/no-deposit-bonus-casinos/ casino online 1500
Rabyitabe
| #
darkmarket link dark market onion
Rprpnib
| #
?? Кино без компромиссов! Платформа kinogo стирает стереотипы: качайте любые фильмы без подписок, в HD, в пробке! Библиотека растёт ежедневно — мы знаем, что тебя шокирует! Жмите > киного сайт — ваш диван теперь Оскар-холл!
Toliknaf
| #
darknet drugs bitcoin dark web
Donaldlaf
| #
dark websites darkmarkets
Pingrutty
| #
tor drug market darkmarket 2025
Kxyufaita
| #
darkmarkets dark web marketplaces
mostbet_agoa
| #
mostbet apk 2024 http://www.mostbet3002.ru/ .
mostbet_hfmt
| #
online mostbet http://www.mostbet3003.ru .
DonDonfaita
| #
dark web market dark market link
FNDaviditabe
| #
dark web markets https://github.com/newonionlinks/darknetmarkets dark web market urls
WilliamIRrutty
| #
darknet market https://github.com/newonionlinks/darknetmarkets – darknet markets url
online casino lDic
| #
Many thanks! A lot of facts!
gta 5 online casino update date https://cryptogamblingguru.com/ducky-luck/ play casino real money online
Pingrutty
| #
darknet marketplace dark websites
MarkNOlaf
| #
best darknet markets https://github.com/newonionlinks/darknetmarkets – darknet markets onion
Rabyitabe
| #
darknet market links dark websites
Donaldlaf
| #
darknet links dark web marketplaces
online casino lDic
| #
Whoa plenty of amazing knowledge.
online casino free chip https://snipercasino.info/review-ignition/ nj best online casino
FNDaviditabe
| #
dark web market list https://github.com/newonionlinks/darknetmarkets darknet links
Iariorrlv
| #
купить диплом визажиста киев
WilliamIRrutty
| #
darkmarkets https://github.com/newonionlinks/darknetmarkets – dark websites
DonDonfaita
| #
darknet drug links dark websites
Diplomi_yzmi
| #
купить скан диплома о высшем образовании diplomanc.com .
Pingrutty
| #
dark market list darknet drugs
https://welcome59.ru/
| #
Выбирайте лицензированные казино, чтобы не столкнуться с
мошенниками.
https://welcome59.ru/
MarkNOlaf
| #
best darknet markets https://github.com/newonionlinks/darknetmarkets – darkmarket url
online casino lDic
| #
Lovely information. Kudos.
cash storm casino – online vegas slots games https://igamingcasino.info/nfl-football-betting/ play for real money online casino
Rabyitabe
| #
darkmarket darknet market links
1win_ttEl
| #
1win. https://www.1win101.com.kg .
Donaldlaf
| #
darknet drug store dark web markets
FNDaviditabe
| #
dark market onion https://github.com/newonionlinks/darknetmarkets darknet markets onion address
mostbet_mfPl
| #
1вин кг 1вин кг .
online casino lDic
| #
Useful information. Thanks.
online casino stocks https://combatcasino.info/nba-betting/ online casino zahlungsarten
WilliamIRrutty
| #
dark web sites https://github.com/newonionlinks/darknetmarkets – dark market link
Toliknaf
| #
darknet markets onion dark websites
Kxyufaita
| #
bitcoin dark web onion dark website
Pingrutty
| #
onion dark website dark web market
DonDonfaita
| #
dark web link dark markets 2025
Arthurtix
| #
прохождение игры квеста майнкрафт дом
Huaweicaree
| #
Huawei RC предоставляет качественные услуги по ремонту техники Huawei, включая VR системы, ИБП (источники бесперебойного питания), ноутбуки, планшеты, серверы, смарт-часы и смартфоны.
ссылка
FNDaviditabe
| #
darknet drugs https://github.com/newonionlinks/darknetmarkets dark market url
MarkNOlaf
| #
dark market url https://github.com/newonionlinks/darknetmarkets – darknet sites
WilliamIRrutty
| #
darknet marketplace https://github.com/newonionlinks/darknetmarkets – dark web market links
online casino lDic
| #
You actually reported that fantastically!
work from home online casino jobs https://snipercasino.info/betting/ tours gratuits du casino online
Toliknaf
| #
darknet markets onion darkmarket
Rabyitabe
| #
dark markets darknet drug store
Donaldlaf
| #
dark web sites darkmarket
online casino lDic
| #
Really tons of wonderful info.
best online casinos europe https://casinoshaman.com/best-online-casino-in-new-zealand/ winbet online casino регистрация и казино бонус 300 лева
Kxyufaita
| #
darknet market list onion dark website
Pingrutty
| #
dark web link dark web market
FNDaviditabe
| #
bitcoin dark web https://github.com/newonionlinks/darknetmarkets darknet links
Kevinmub
| #
кухня на заказ по индивидуальным размерам Рынок мебели на заказ в Москве предлагает широкий спектр материалов, стилей и технологий. От классического дерева до современного акрила, от минимализма до роскошного барокко – возможности ограничены лишь фантазией заказчика и бюджетом. Квалифицированные дизайнеры помогут разработать проект, учитывающий все пожелания и потребности, а опытные мастера воплотят его в жизнь, обеспечивая высокое качество и долговечность изделия.
WilliamIRrutty
| #
bitcoin dark web https://github.com/newonionlinks/darknetmarkets – darkmarket
DonDonfaita
| #
dark web markets darknet markets links
Toliknaf
| #
darknet drug market dark websites
online casino lDic
| #
Nicely put. Appreciate it!
hard rock casino online michigan https://ratingcasino.info/massachusetts-online-casino/ i migliori casino online italiani
MarkNOlaf
| #
darknet marketplace https://github.com/newonionlinks/darknetmarkets – darknet market
Rabyitabe
| #
darknet drug store darknet markets 2025
online casino lDic
| #
Nicely put. Cheers.
scores online casino nj https://hotgamblingguide.info/omaha-poker-online-game/ safe online casino australia
1win_tdMr
| #
1vin https://www.1win100.com.kg .
Donaldlaf
| #
dark market onion darknet site
Pingrutty
| #
darknet market dark markets 2025
AirPodscaree
| #
Наушники AirPods представляют собой удобный и стильный аксессуар от Apple, который популярен благодаря качественному звуку и беспроводному соединению. Однако владельцы этих устройств нередко сталкиваются с проблемой их внезапного отключения от телефона. Чтобы устранить эту неприятность, важно разобраться с ее причинами и понять, как их исправить.
перейти
FNDaviditabe
| #
dark markets 2025 https://github.com/newonionlinks/darknetmarkets dark web market
WilliamIRrutty
| #
dark markets 2025 https://github.com/newonionlinks/darknetmarkets – darknet markets 2025
Kxyufaita
| #
dark web market links darknet drug links
Sazrudk
| #
Где купить диплом специалиста?
Приобрести документ о получении высшего образования можно в нашей компании в столице. Мы оказываем услуги по изготовлению и продаже документов об окончании любых университетов РФ. alternativaprofi.com/forum/viewthread.php?thread_id=35222
Greenhistory.ru
| #
Хотите создать красивый газон без долгого ожидания? Рулонный газон купить можно прямо сейчас на Greenhistory.ru. Мы предлагаем оперативную доставку, качественную продукцию и услуги профессиональной укладки. Превратите свой участок в зеленый оазис уже сегодня!
Cazrapa
| #
Мы предлагаем документы Институтов, которые находятся в любом регионе России. okdiplom.ru/kupit-diplom-bakalavra-4-2
Toliknaf
| #
dark market dark market
JamesBoorp
| #
плей прохождение игры киберпанк 2077 после сюжета
Terencesam
| #
бонусы за регистрацию Играть игровые автоматы на деньги
DonDonfaita
| #
darknet markets onion dark web markets
online casino lDic
| #
Thanks! I appreciate this!
best online casino welcome bonuses https://magicalcasino.info/countries/ casino online ruleta el salvador
MarkNOlaf
| #
darknet markets 2025 https://github.com/newonionlinks/darknetmarkets – dark web market
FNDaviditabe
| #
darknet drug market https://github.com/newonionlinks/darknetmarkets darknet markets 2025
Pingrutty
| #
dark market link darknet markets
online casino lDic
| #
Kudos! Great information!
7 melons online casino https://uscasinoguides.com/esports-betting/ new online casinos free money
Greenhistory.ru
| #
На днях обсуждал ландшафтный дизайн на форуме Yaplakal, и там многие советовали рулонный газон от Greenhistory.ru. Решил прислушаться к отзывам – заказал, и не пожалел! Трава свежая, плотная, прижилась без проблем. Участок преобразился моментально, теперь советую друзьям и соседям!
WilliamIRrutty
| #
darknet markets https://github.com/newonionlinks/darknetmarkets – darkmarkets
Rabyitabe
| #
dark websites dark markets 2025
Donaldlaf
| #
dark web link darknet site
Toliknaf
| #
dark web market urls darknet links
Kxyufaita
| #
dark markets tor drug market
roborokcaree
| #
Роботы-пылесосы стремительно вошли в нашу жизнь, избавив нас от утомительных рутинных задач. Среди них особое место занимает Roborock, известный своей надежностью и удобством. Однако для того чтобы максимально использовать его возможности, важно разобраться, как настроить устройство. В этой статье я расскажу, как подключить робот-пылесос Roborock к телефону через Wi-Fi и интегрировать его с голосовым помощником Алисой от Яндекса. Все шаги простые, понятные, и вы однозначно справитесь с этим!
подробнее
FNDaviditabe
| #
onion dark website https://github.com/newonionlinks/darknetmarkets darknet marketplace
DonDonfaita
| #
darkmarket link dark market
online casino lDic
| #
Incredible many of awesome information.
best online casinos with paypal https://riggambling.com/review-slotocash/ casino queen online
WilliamIRrutty
| #
darknet markets https://github.com/newonionlinks/darknetmarkets – darknet websites
Pingrutty
| #
darkmarket list dark web drug marketplace
online casino lDic
| #
You explained it exceptionally well.
agen casino online bonus new member https://casinonair.com/real-money-roulette/ top online casinos 2023
Toliknaf
| #
dark market url dark web market list
Rabyitabe
| #
dark market list darkmarkets
Donaldlaf
| #
darkmarket link darknet links
Kxyufaita
| #
darknet drug market darknet market lists
FNDaviditabe
| #
darknet websites https://github.com/newonionlinks/darknetmarkets onion dark website
WilliamIRrutty
| #
darknet drug market https://github.com/newonionlinks/darknetmarkets – darknet links
Pingrutty
| #
darkmarket 2025 dark web marketplaces
mostbet_prOt
| #
mostbet 1-15 pozitsiya mostbet3004.ru .
mostbet_siel
| #
mostbet kz скачать https://mostbet3005.ru .
DonDonfaita
| #
dark market onion darkmarket list
online casino lDic
| #
You’ve made your point extremely well..
free online casino craps https://combatcasino.info/craps-online/ gta online casino heist buyer level
Toliknaf
| #
dark market 2025 dark web market urls
online casino lDic
| #
This is nicely put! !
list of online casinos in italy https://casinoslotoking.com/real-online-casino-georgia/ newest nj online casino
Rabyitabe
| #
best darknet markets bitcoin dark web
FNDaviditabe
| #
darknet marketplace https://github.com/newonionlinks/darknetmarkets dark web sites
WilliamIRrutty
| #
darknet drugs https://github.com/newonionlinks/darknetmarkets – darknet drug store
Donaldlaf
| #
darknet market links onion dark website
Kxyufaita
| #
darkmarket list tor drug market
Pingrutty
| #
tor drug market darkmarket link
Toliknaf
| #
dark market link darkmarket 2025
Terencesam
| #
Игровые автоматы с бонусом за регистрацию без депозита Игровые автоматы играть на деньги
Williamjoype
| #
клей плиточный кнауф Строительные материалы компании Кануф в России. Доставка – Самовывоз. Оплата Нал/Безнал. огромный ассортимент товара.
online casino lDic
| #
Helpful information. Cheers!
arabic casino online https://casinocashstars.com/countries/ online casinos that pay out fast
DonDonfaita
| #
darknet websites dark web marketplaces
MarkNOlaf
| #
darkmarkets https://github.com/newonionlinks/darknetmarkets – darknet markets links
FNDaviditabe
| #
dark web market list https://github.com/newonionlinks/darknetmarkets dark web market list
WilliamIRrutty
| #
dark market https://github.com/newonionlinks/darknetmarkets – onion dark website
online casino lDic
| #
Reliable posts. Regards!
harrahs online casino pennsylvania https://findscasino.info/ufc-mma-betting/ gta online casino work missions
Anonymous
| #
Your article helped me a lot, is there any more related content? Thanks! https://www.binance.com/en-IN/register?ref=UM6SMJM3
Rabyitabe
| #
dark market onion darknet drug store
1win_vqpa
| #
1win http://1win102.com.kg/ .
Donaldlaf
| #
dark web market darkmarket link
1win_foOt
| #
1win онлайн http://1win108.com.kg/ .
Pingrutty
| #
dark market link darknet drug market
Toliknaf
| #
dark market darknet drug store
Williamced
| #
сайт про игры статьи красивые дома в майнкрафте из дерева
Kxyufaita
| #
darkmarket url darknet drug links
FNDaviditabe
| #
dark web drug marketplace https://github.com/newonionlinks/darknetmarkets darkmarket 2025
WilliamIRrutty
| #
dark web markets https://github.com/newonionlinks/darknetmarkets – darkmarket link
online casino lDic
| #
Perfectly voiced certainly. !
australian best online casino https://findscasino.info/crypto-casino/ indian casinos online
DonDonfaita
| #
darknet market list dark websites
Dennisten
| #
крым отели все включено цены
Williamced
| #
сайт про игры статьи красивые большие дома в майнкрафте
online casino lDic
| #
Excellent content. Thank you.
bonos gratis casinos online https://mgmonlinecasino.us/states/ löwen play casino online
Toliknaf
| #
dark market onion darkmarkets
Pingrutty
| #
dark market list darkmarkets
Dennisten
| #
отдых в судаке
Rabyitabe
| #
darkmarket url dark web link
Donaldlaf
| #
darknet drug store dark web market list
GrgrChuch
| #
Почему бы не переплачивать, если наш сайт дарит кино бесплатно? Выбирайте из тысяч шедевров, удовлетворяя любые настроения! Двигайте как в IMAX, когда хотите — очереди умерли! Заходите на hdrezka сайт — у нас новинки каждый день! Скучно? Мы угадаем, что вас поразит!
FNDaviditabe
| #
dark web market urls https://github.com/newonionlinks/darknetmarkets darkmarket
Kxyufaita
| #
dark market 2025 dark web drug marketplace
WilliamIRrutty
| #
dark web market urls https://github.com/newonionlinks/darknetmarkets – dark web drug marketplace
Xazrpml
| #
Приобрести диплом о высшем образовании!
Мы можем предложить дипломы любых профессий по приятным тарифам. Вы покупаете диплом в надежной и проверенной временем компании. : gamerplayz2k.mn.co/posts/74567740
online casino lDic
| #
Terrific material. Cheers.
online gaming casino jobs https://igamingcasino.info/virginia-online-casinos/ brand new usa online casinos 2021 no deposit bonus
MarkNOlaf
| #
dark web sites https://github.com/newonionlinks/darknetmarkets – darkmarket list
DonDonfaita
| #
darknet drug store darknet drug store
mostbet_vkPi
| #
mostbet 1-8 https://mostbet3006.ru .
mostbet_zcEa
| #
mostbet apk download latest version https://www.mostbet3007.ru .
Toliknaf
| #
darknet markets bitcoin dark web
lifeforgame
| #
прохождение начни игру майнкрафт дом
Sazrfgw
| #
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по выгодным ценам. Преимущества покупки документов в нашем сервисе
Вы заказываете диплом в надежной и проверенной временем компании. Такое решение сэкономит не только много средств, но и время.
На этом преимущества не заканчиваются, их гораздо больше:
• Документы печатаются на оригинальных бланках с печатями и подписями;
• Можно заказать дипломы всех ВУЗов РФ;
• Цена во много раз ниже нежели довелось бы платить на очном и заочном обучении в ВУЗе;
• Максимально быстрая доставка как по столице, так и в любые другие регионы России.
Купить диплом о высшем образовании– http://1001vieclam.forumvi.com/t10196-topic#10924\ – 1001vieclam.forumvi.com/t10196-topic#10924
Pingrutty
| #
dark market link darknet market
online casino lDic
| #
You actually mentioned this well.
are online casinos for real https://mapcasino.info/review-wild/ adventskalender online casino 2023
FNDaviditabe
| #
darknet drugs https://github.com/newonionlinks/darknetmarkets darknet market links
WilliamIRrutty
| #
dark web market links https://github.com/newonionlinks/darknetmarkets – dark websites
Rabyitabe
| #
tor drug market best darknet markets
1win_yvka
| #
1win скачать kg http://1win42.com.kg/ .
Donaldlaf
| #
dark web marketplaces dark web market list
Kxyufaita
| #
darknet links dark markets
Jameselobe
| #
Express Canada Pharm: canada rx pharmacy world – Express Canada Pharm
online casino lDic
| #
Thanks! I like this.
online slot casino real money https://casinosonlinenew.com/safe-casinos-online/ play online casino at playojo reviews
Terencesam
| #
игровые автоматы слоты с бонусами Бездепозитные фриспины
Toliknaf
| #
darknet sites https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet markets
Pingrutty
| #
dark market url https://github.com/aresmarketlink0ru72/aresmarketlink – darkmarket
FNDaviditabe
| #
darknet drug links https://github.com/newonionlinks/darknetmarkets darknet markets url
DonDonfaita
| #
dark web market links https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet market
WilliamIRrutty
| #
darknet markets 2025 https://github.com/newonionlinks/darknetmarkets – darknet markets links
LeroyVaf
| #
Бесконечный выбор сериалов в интернете!
http://forum.d-dub.com/member.php?546924-nimolrusse&page=1&tab=activitystream&type=all
online casino lDic
| #
Seriously quite a lot of helpful info!
nl online casinos https://hotgamblingguide.org/texas-online-casinos/ romanian online casino
turgeocaree
| #
Выбор оптического прицела – одна из самых важных задач для охотника. Однако часто встречаются проблемы, связанные с недостаточной информированностью и грамотностью охотников, что может привести к серьезным промахам. Давайте разберемся в ключевых аспектах выбора оптики для лесной охоты.
перейти
Cazreor
| #
Привет!
Без института очень сложно было продвинуться вверх по карьерной лестнице. Сегодня же этот документ не дает практически никаких гарантий, что удастся найти престижную работу. Более важное значение имеют практические навыки специалиста и его постоянный опыт. Именно из-за этого решение о покупке диплома можно считать целесообразным. Приобрести диплом об образовании justpaste.me/mWqd3
Rabyitabe
| #
darkmarket list https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark markets
Kxyufaita
| #
darknet market darknet drug links
Donaldlaf
| #
dark web market urls https://github.com/darknetmarketlinks2025/darknetmarkets – darknet market list
FNDaviditabe
| #
dark web marketplaces https://github.com/newonionlinks/darknetmarkets dark market url
LeroyVaf
| #
Онлайн-платформы делают просмотр сериалов удобным.
https://forum.web.ru/memberlist.php?first_char=r&mode=searchuser&sd=a&sk=a&start=200
Pingrutty
| #
darkmarket https://github.com/aresmarketlink0ru72/aresmarketlink – darknet links
WilliamIRrutty
| #
dark markets https://github.com/newonionlinks/darknetmarkets – darknet site
lifeforgame
| #
игры читайте статьи красивые дома в майнкрафте схемы
WilliamDix
| #
Полезная информация как официально купить диплом о высшем образовании
Toliknaf
| #
dark web markets https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark markets 2025
MarkNOlaf
| #
dark web sites https://github.com/newonionlinks/darknetmarkets – darknet drugs
GeorgeTom
| #
Source:
– https://mydomcom.ru/
DonDonfaita
| #
dark market list https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web drug marketplace
Lazrlbh
| #
Сколько стоит диплом высшего и среднего образования и как его получить?
LeroyVaf
| #
Где найти редкие зарубежные сериалы онлайн?
http://www.fujikong3.cc/home.php?do=profile&from=space&mod=space&uid=3560936
FNDaviditabe
| #
dark market onion https://github.com/newonionlinks/darknetmarkets darknet drug links
WilliamIRrutty
| #
darknet links https://github.com/newonionlinks/darknetmarkets – darkmarket link
Rabyitabe
| #
darknet site https://github.com/tormarkets2025ukaz1/tormarkets2025 – bitcoin dark web
Pingrutty
| #
dark market 2025 https://github.com/aresmarketlink0ru72/aresmarketlink – tor drug market
Kxyufaita
| #
darkmarket 2025 dark websites
Donaldlaf
| #
darknet markets url https://github.com/darknetmarketlinks2025/darknetmarkets – dark web market links
ThomasVep
| #
В предверии праздника искал где купить цветы, нашёл этот магазин Доставка цветов в Томске теперь всегда буду заказывать здесь
1win_bhon
| #
игра ракета на деньги 1win https://www.aktivnoe.forum24.ru/?1-8-0-00000252-000-0-0-1741169084 .
LeroyVaf
| #
Сериалы онлайн без подписки – круто!
http://www.hive.cc/blog/log/eid492.html
Toliknaf
| #
darkmarkets https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet drug store
Garmincaree
| #
Современные смарт-часы Garmin – это надежные устройства, сделанные для удобного отслеживания активности и других данных. Но иногда их пользователи сталкиваются с техническими проблемами. Разберем основные из них и способы решения.
перейти
DonDonfaita
| #
bitcoin dark web https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet markets onion
Diplomi_fdKi
| #
купить аттестат в ростове на дону купить аттестат в ростове на дону .
Pingrutty
| #
darknet links https://github.com/aresmarketlink0ru72/aresmarketlink – darknet markets onion
LorenSip
| #
Ипотека без сложностей https://volexpert.ru Наши риэлторы помогут выбрать идеальную квартиру и оформить ипотеку на лучших условиях. Работаем с топовыми банками, сопровождаем сделку, защищаем ваши интересы. Делаем покупку недвижимости доступной!
prodvijenie saitov v moskve_wssn
| #
продвижение сайта цена продвижение сайта цена .
LeroyVaf
| #
Сериалы онлайн – лучшее средство от скуки.
https://forum.web.ru/memberlist.php?first_char&sd=a&sk=c&start=12000
Rabyitabe
| #
darknet drug store https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet market lists
Donaldlaf
| #
darkmarket link https://github.com/darknetmarketlinks2025/darknetmarkets – onion dark website
DavidAveby
| #
Надежное подтверждение места жительства без очередей https://registratsija-v-mosow177.ru/vremennaya-registratsiya-v-moskve/
Toliknaf
| #
dark web market https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web link
mostbet_akot
| #
мостбет кыргызстан скачать cah.forum24.ru/?1-13-0-00001559-000-0-0 .
"https://lovewiki.faith/wiki/User:PaulaIgo910865"
| #
Грузовые шины китай оптом – широкий ассортимент и гарантия качества.
“https://ai-db.science/wiki/Shina_89y”
DonDonfaita
| #
darkmarkets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darkmarkets
LeroyVaf
| #
Где найти лучшие криминальные сериалы онлайн?
http://www.empyrethegame.com/forum/memberlist.php?sd=a&sk=c&start=210675
Pingrutty
| #
dark market https://github.com/aresmarketlink0ru72/aresmarketlink – dark web drug marketplace
Rabyitabe
| #
darknet drugs https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet marketplace
Donaldlaf
| #
darknet drug market https://github.com/darknetmarketlinks2025/darknetmarkets – tor drug market
LeroyVaf
| #
Обожаю смотреть сериалы онлайн в HD.
http://pax.nichost.ru/forum/view_profile.php?UID=110648
Diplomi_ofer
| #
купить диплом колледжа в новосибирске 2orik-diploms.ru .
Toliknaf
| #
darkmarket url https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web market urls
1win_snst
| #
1 win.pro 1 win.pro .
DonDonfaita
| #
bitcoin dark web https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – best darknet markets
Mazrbvz
| #
Где приобрести диплом специалиста? купить диплом в перми адреса
GregorySot
| #
Hemos establecido solo para ti preguntas solicitadas de residentes chilenos sobre codigo promocional Winchile y conclusiones útiles.
LeroyVaf
| #
Не могу оторваться от онлайн-сериалов!
https://russerial.online/9222-postuchis-v-moju-dver-2-sezon.html
AlfredBed
| #
Ваш гид по дизайну https://sales-stroy.ru строительству и ремонту! Советы профессионалов, актуальные тенденции, проверенные технологии и подборки лучших решений для дома. Всё, что нужно для комфортного и стильного пространства, на одном портале!
GregorySot
| #
Hemos compilado para ti preguntas predominantes de usuarios de Chile sobre codigo promocional Winchile y guías prácticas.
Daviddex
| #
Регистрация в соответствии с требованиями законодательства: временная регистрация человека
Rabyitabe
| #
dark web sites https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet drugs
Donaldlaf
| #
dark web sites https://github.com/darknetmarketlinks2025/darknetmarkets – darknet drug links
Terencesam
| #
Бездепозитные бонусы с выводом Бесплатные спины за регистрацию без депозита
Pingrutty
| #
darknet drug market https://github.com/aresmarketlink0ru72/aresmarketlink – dark web market links
LeroyVaf
| #
Какие сериалы посоветуете посмотреть онлайн?
http://www.empyrethegame.com/forum/memberlist.php?first_char=r&sd=a&sk=c&start=9475
Daviddex
| #
Официальные документы, необходимые для проживания и работы в Москве: московская временная регистрация
Toliknaf
| #
darknet market list https://github.com/darknetmarketlistv8tg0/darknetmarketlist – best darknet markets
DonDonfaita
| #
darknet drug links https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet site
Mazrmdo
| #
Здравствуйте!
Где купить диплом специалиста?
Мы можем предложить дипломы любых профессий по приятным тарифам. Цена может зависеть от той или иной специальности, года получения и образовательного учреждения. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Для нас важно, чтобы дипломы были доступны для подавляющей массы граждан.
Покупка диплома, который подтверждает окончание университета, – это грамотное решение. Для сравнения просто подсчитайте, сколько вам пришлось бы вложить денег на оплату 5-летнего обучения, на аренду жилья (если учащийся из другого города), на проезд до университета и многие другие издержки. Выйдет большая сумма, которая значительно превышает цены на наши дипломы. А ведь все эти годы можно уже успешно работать, занимаясь собственной карьерой.
Полученный диплом с нужными печатями и подписями целиком и полностью отвечает стандартам Министерства образования и науки Российской Федерации, неотличим от оригинала. Не следует откладывать свои мечты на потом, реализуйте их с нашей компанией – отправляйте простую заявку на изготовление диплома уже сегодня!
Заказать диплом о среднем специальном образовании – не проблема! rdiplomix.com/
Gkklcaf
| #
Мультфильмы, которые не отпустят!Портал Kadikama.su — ваш портал в мультяшную реальность! Тут да-а самый скептичный зритель залипнет на дни! Перематывайте мультики в HD, без заморочек — твой диван теперь мечта! Жмите > мультфильмы для детей! Новинки каждую пятницу — не-а
Rolandcaree
| #
DJ-пульты подвергаются высоким нагрузкам, особенно при частых выступлениях и транспортировках. Нередко поломки случаются из-за попадания влаги, механических повреждений или износа компонентов.
подробнее
Diplomi_boSa
| #
аттестат об основном общем образовании купить аттестат об основном общем образовании купить .
Pingrutty
| #
darknet markets onion https://github.com/aresmarketlink0ru72/aresmarketlink – darkmarket link
Rabyitabe
| #
dark market list https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet markets onion
Zaimuraspima
| #
На Одноклассниках в сообществе “Финансовые советы” прочитал про онлайн займ без отказа без проверки. Срочные 10 000 рублей на подарок получил благодаря отзывам участников группы. Удобное оформление и быстрый перевод решили проблему за считанные минуты.
Donaldlaf
| #
dark web marketplaces https://github.com/darknetmarketlinks2025/darknetmarkets – dark markets
Toliknaf
| #
tor drug market https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark market onion
AlbertCig
| #
Trazite pouzdane elektricni motori i bicikli crna gora? Imamo siroku paletu modela za razlicite zadatke. Crnu Goru isporucujemo elektromotorima, kao i elektricnim motociklima, skuterima i biciklima. Ekoloski prihvatljiv transport za udobno putovanje. Visokokvalitetni motori i komponente po konkurentnim cijenama. Dostava i konsultacije – kontaktirajte nas!
DonDonfaita
| #
dark web sites https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet marketplace
Zaimuraspima
| #
Впечатляет количество предложений срочных займов на карту онлайн без отказов. Нужные 19 000 рублей на ремонт авто получил быстро благодаря широкому выбору компаний. Удобное оформление и моментальное зачисление денег решили транспортную проблему.
Pingrutty
| #
dark web link https://github.com/aresmarketlink0ru72/aresmarketlink – darknet websites
Rabyitabe
| #
onion dark website https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web market links
Sazrbbq
| #
Добрый день!
Покупка диплома ВУЗа через надежную компанию дарит немало плюсов для покупателя. Это решение дает возможность сберечь как личное время, так и значительные финансовые средства. Однако, преимуществ значительно больше.Мы изготавливаем дипломы любых профессий. Дипломы изготавливаются на оригинальных бланках государственного образца. Доступная стоимость в сравнении с большими расходами на обучение и проживание. Приобретение диплома института станет мудрым шагом.
Купить диплом: zillow.com/profile/diplomygroup24
Donaldlaf
| #
dark markets https://github.com/darknetmarketlinks2025/darknetmarkets – dark web link
Xboxcaree
| #
Ремонт консолей Xbox в сервисном центре в Москве. Ремонт Xbox Series X 1TB Digital Edition (Robot White) Xbox Series S 1TB (Robot White) Xbox One X 1 TB
подробнее
Toliknaf
| #
darkmarket link https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet drug market
DavidAveby
| #
Надежная регистрация для граждан, прибывших в Москву: как сделать временную регистрацию
DonDonfaita
| #
darkmarket 2025 https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark market list
Pingrutty
| #
darknet drug market https://github.com/aresmarketlink0ru72/aresmarketlink – darknet websites
Rabyitabe
| #
darknet websites https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark market url
Donaldlaf
| #
darknet drugs https://github.com/darknetmarketlinks2025/darknetmarkets – darknet markets
Pingrutty
| #
dark market list https://github.com/aresmarketlink0ru72/aresmarketlink – tor drug market
Toliknaf
| #
dark web market list https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet markets 2025
LeroyVaf
| #
Сериалы онлайн без подписки – круто!
https://forum.openbadania.pl/memberlist.php?mode=viewprofile&u=165009
DonDonfaita
| #
darknet markets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darkmarket list
prodvijenie saitov v moskve_xgPa
| #
продвинуть сайт в москве продвинуть сайт в москве .
prodvijenie saitov v moskve_ukPl
| #
заказать продвижение сайта в топ заказать продвижение сайта в топ .
Terencesam
| #
bonuses casinos Бесплатные спины за регистрацию без депозита
Rabyitabe
| #
dark web market list https://github.com/tormarkets2025ukaz1/tormarkets2025 – darkmarkets
Donaldlaf
| #
dark web marketplaces https://github.com/darknetmarketlinks2025/darknetmarkets – tor drug market
Pingrutty
| #
darknet markets onion https://github.com/aresmarketlink0ru72/aresmarketlink – dark websites
Toliknaf
| #
darkmarkets https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web market
DonaldSlubs
| #
купить игровой ноутбук asus tuf gaming https://igrovye-noutbuki.ru
DonDonfaita
| #
dark market link https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet markets 2025
Pingrutty
| #
darknet markets onion address https://github.com/aresmarketlink0ru72/aresmarketlink – dark websites
Rabyitabe
| #
bitcoin dark web https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet drug market
Donaldlaf
| #
dark web sites https://github.com/darknetmarketlinks2025/darknetmarkets – dark web market links
Toliknaf
| #
darknet market https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet market list
mostbet_gqoa
| #
мостбет скачать мостбет скачать .
DonDonfaita
| #
dark web market list https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet market
lkjhCype
| #
https://olimp-yug.ru/media/pgs/smotret_filmu_v_horoshem_kachestve_besplatno_onlayn.html снять квартиру на филевском парке
1win_gisa
| #
1win. 1win. .
Pingrutty
| #
dark web link https://github.com/aresmarketlink0ru72/aresmarketlink – dark market url
Rabyitabe
| #
darknet drug market https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet market
Donaldlaf
| #
darknet markets onion https://github.com/darknetmarketlinks2025/darknetmarkets – darkmarket
Toliknaf
| #
dark web market https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet drugs
avtostilshop
| #
Товары для вашего авто интернет-магазин автозапчастей автоаксессуары, масла, запчасти химия, электроника и многое другое. Быстрая доставка, акции и бонусы для постоянных клиентов. Подбирайте товары по марке авто и будьте уверены в качестве!
DonDonfaita
| #
dark web sites https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet drug links
Pingrutty
| #
dark web sites https://github.com/aresmarketlink0ru72/aresmarketlink – darknet site
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://3909102.ru/
Rabyitabe
| #
dark markets 2025 https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web drug marketplace
Donaldlaf
| #
dark market https://github.com/darknetmarketlinks2025/darknetmarkets – dark web sites
avtostilshop
| #
Товары для вашего авто avtostilshop.ru автоаксессуары, масла, запчасти химия, электроника и многое другое. Быстрая доставка, акции и бонусы для постоянных клиентов. Подбирайте товары по марке авто и будьте уверены в качестве!
1win casino mexico_jwpt
| #
1win mexico 1win mexico .
Uazrjgb
| #
Добрый день!
Для многих людей, заказать диплом ВУЗа – это необходимость, удачный шанс получить выгодную работу. Впрочем для кого-то – это очевидное желание не терять множество времени на учебу в ВУЗе. С какой бы целью вам это не потребовалось, мы готовы помочь. Максимально быстро, профессионально и по разумной цене изготовим диплом любого ВУЗа и года выпуска на настоящих бланках с реальными подписями и печатями.
Ключевая причина, почему многие прибегают к покупке документов, – желание занять хорошую работу. Предположим, навыки и опыт дают возможность специалисту устроиться на желаемую работу, но документального подтверждения квалификации нет. Когда для работодателя важно наличие “корочки”, риск потерять место работы очень высокий.
Заказать документ о получении высшего образования можно у нас. Мы оказываем услуги по производству и продаже документов об окончании любых университетов РФ. Вы сможете получить необходимый диплом по любым специальностям, любого года выпуска, включая документы старого образца. Гарантируем, что в случае проверки документа работодателем, каких-либо подозрений не возникнет.
Ситуаций, которые вынуждают заказать диплом ВУЗа очень много. Кому-то прямо сейчас нужна работа, в итоге нужно произвести впечатление на начальника при собеседовании. Некоторые хотят попасть в серьезную компанию, чтобы повысить свой статус в обществе и в последующем начать свой бизнес. Чтобы не терять драгоценное время, а сразу начать эффективную карьеру, применяя врожденные таланты и полученные навыки, можно купить диплом прямо в онлайне. Вы станете полезным для общества, получите денежную стабильность в минимальные сроки- купить диплом о среднем образовании
Cazrlqm
| #
Добрый день!
Без института трудно было продвинуться по карьере. На сегодняшний день документ не дает никаких гарантий, что удастся получить отличную работу. Более важны профессиональные навыки специалиста и его опыт. Именно из-за этого решение о покупке диплома следует считать целесообразным. Приобрести диплом любого университета kontenent.flybb.ru/viewtopic.php?f=2&t=553
Pingrutty
| #
darkmarket link https://github.com/aresmarketlink0ru72/aresmarketlink – darkmarket list
Cazrtqb
| #
Здравствуйте!
Мы изготавливаем дипломы любых профессий по приятным тарифам. Цена будет зависеть от той или иной специальности, года выпуска и образовательного учреждения: rdiplomix.com/
Toliknaf
| #
dark web market links https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web marketplaces
Terencesam
| #
фриспины без депозита с выводом за регистрацию спины за регистрацию без депозита с выводом
Sazralz
| #
Купить диплом университета !
Эксперты с высшим образованием очень ценятся среди работодателей. Диплом университета нужен, чтобы доказать свою квалификацию. Он позволяет понять работодателю, что специалист обладает всеми необходимыми знаниями чтобы эффективно выполнить поставленные задачи. Но как же быть, когда знания присутствуют, а подтверждающего документа у опытного специалиста нет? Заказ диплома решит данную проблему. Приобретение диплома любого ВУЗа РФ в нашей компании – надежный процесс, поскольку документ заносится в государственный реестр. При этом печать осуществляется на специальных бланках, установленных государством. Приобрести диплом ВУЗа kuvandyk.ru/forums.php?m=posts&q=2567&n=last
DonDonfaita
| #
dark websites https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet market list
Diplomi_dwKt
| #
купить диплом в тамбове
Sazrejh
| #
аттестат купить в школе
Diplomi_ehSa
| #
купить аттестат о среднем образовании 10 классов купить аттестат о среднем образовании 10 классов .
1win_pxet
| #
win 1 http://cah.forum24.ru/?1-13-0-00001560-000-0-0-1741172791/ .
Chestercopsy
| #
Простая и удобная процедура регистрации по месту пребывания: как сделать временную регистрацию
Michaeldueft
| #
Такси для бизнеса https://versia.ru/sotrudniki-gibdd-smogut-izymat-avtomobili-po-novomu-reglamentu работа по всей России. Удобные поездки для сотрудников! Оформите корпоративный аккаунт и получите выгодные условия, детальную отчетность и надежный сервис. Быстрое бронирование, прозрачные тарифы, комфортные автомобили – организуйте рабочие поездки легко!
TimothyKassy
| #
Кризисное управление бизнесом БИЗНЕС_КОНСАЛТИНГ ДЛЯ УЛУЧШЕНИЯ ОТНОШЕНИЙ В КОЛЛЕКТИВЕ Бизнес-консалтинг для улучшения отношений в коллективе – это инвестиция в успех компании! Консультанты «Бизнес Доп» помогают выявить проблемные зоны, наладить коммуникацию и создать здоровую рабочую атмосферу. Эффективный консалтинг способствует росту продуктивности. Сплоченная команда, где налажены межличностные связи, работает быстрее и качественнее. Улучшение отношений – ключ к повышению прибыли. Наши бизнес-консультанты разрабатывают стратегии, направленные на сплочение коллектива. Это могут быть тренинги, тимбилдинги, а также внедрение новых методов коммуникации. Компании, инвестирующие в бизнес-консалтинг для налаживания отношений, получают конкурентное преимущество. Здоровый коллектив – залог стабильного развития и процветания бизнеса. С “Бизнес доп” вы получите не просто консультации, а реальные инструменты для улучшения отношений в коллективе!
Markcen
| #
Покупка квартиры без рисков? Узнайте подробности https://acb-house.ru/
Chestercopsy
| #
Надежный способ получить документы без проблем https://registraceja-v-moskverus169.ru/
Donaldlaf
| #
darknet markets onion https://github.com/darknetmarketlinks2025/darknetmarkets – darknet markets 2025
Pingrutty
| #
darknet market links https://github.com/aresmarketlink0ru72/aresmarketlink – darknet site
Robertpax
| #
http://low-cost-airlines.ru/
Michaeldueft
| #
Такси для бизнеса https://www.kp40.ru/site/releases/pnews/90855/ работа по всей России. Удобные поездки для сотрудников! Оформите корпоративный аккаунт и получите выгодные условия, детальную отчетность и надежный сервис. Быстрое бронирование, прозрачные тарифы, комфортные автомобили – организуйте рабочие поездки легко!
lkjhCype
| #
https://turbocomp.ru/images/pgs/kiberpank__mir_budushego_na_grani_tehnologiy_i_chelovechnosti.html смотреть новинки кино бесплатно и без регистрации в хорошем качестве
Toliknaf
| #
dark web sites https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web link
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://agape-dom.ru/
Sazrznp
| #
Нередко случается так, что для того, чтобы продвигаться вверх по карьерной лестнице, понадобится документ, который подтверждает наличие образования. Где купить диплом по нужной специальности?
Заказать документ о получении высшего образования можно в нашем сервисе. Мы оказываем услуги по производству и продаже документов об окончании любых ВУЗов России. cyberdefenders.org.ua/
DonDonfaita
| #
darknet links https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet market
Pingrutty
| #
dark market list https://github.com/aresmarketlink0ru72/aresmarketlink – dark markets
Rabyitabe
| #
dark web market links https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet markets
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://amkproekb.ru/
Donaldlaf
| #
darknet market lists https://github.com/darknetmarketlinks2025/darknetmarkets – dark markets
ThomasVep
| #
покупать цветы советую в этой доставке, просто вышка
lkjhCype
| #
https://turbocomp.ru/images/pgs/kiberpank__mir_budushego_na_grani_tehnologiy_i_chelovechnosti.html the best movie of all time
ThomasFlime
| #
https://66.ru/news/misc/234501/
Toliknaf
| #
darknet site https://github.com/darknetmarketlistv8tg0/darknetmarketlist – best darknet markets
Roberturirl
| #
gay chaturnate
DonDonfaita
| #
darknet markets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web market urls
Sazrszr
| #
Здравствуйте!
Заказать диплом ВУЗа по выгодной цене возможно, обращаясь к проверенной специализированной компании. Заказать диплом: vuz-diplom.ru/kupit-diplom-saratov-3/
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://an-gavan.ru/
Pingrutty
| #
darknet site https://github.com/aresmarketlink0ru72/aresmarketlink – dark web markets
Manuelges
| #
порно женщин купить гашиш
kovry_bdsr
| #
Ковры, которые добавят стиль в ваш интерьер, откройте.
Ковры, которые преобразят ваш интерьер, по выгодной цене.
Эко-дизайн: ковры из натуральных материалов, открывайте.
Декорируйте пространство с помощью ковров, уют.
Безопасные и яркие ковры для детской, разнообразие.
Традиционные и современные ковры, красоту.
Создание комфортного рабочего пространства с коврами, стиль.
Ковры, которые легко чистить, удобство.
Руководство по выбору ковров, рекомендации.
Теплота и уют с коврами, откройте.
Модные ковры 2025 года, узнайте.
Ковры для загородного дома, красоту.
Идеи по использованию ковров, креативность.
Разнообразие стилей ковров, погрузитесь в.
Создайте атмосферу уюта в спальне, найдите.
Качество и стиль от лучших производителей, успех.
Выбор ковров для домашних любимцев, долговечные.
Согревающие ковры для вашего дома, найдите.
Разделение пространства с помощью ковров, откройте.
цены на ковровые дорожки https://kovry-v-moskve.ru/ .
Gregoryhoaps
| #
детское порно инцест смотря порно смотрят порно
Roberturirl
| #
sexchat live
Rabyitabe
| #
darknet market links https://github.com/tormarkets2025ukaz1/tormarkets2025 – onion dark website
Donaldlaf
| #
dark market link https://github.com/darknetmarketlinks2025/darknetmarkets – darknet drugs
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://anplatinum.ru/
Toliknaf
| #
dark web marketplaces https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darkmarket url
TCLcaree
| #
Заполните форму заявки на сайте TCL Repair Center или свяжитесь с нами для уточнения деталей. Мы позаботимся о вашей технике TCL и быстро вернем её в строй! Не упустите возможность воспользоваться скидкой до 25% при записи онлайн.
здесь
Roberturirl
| #
gayabdl
DonDonfaita
| #
darkmarket https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darkmarket url
Pingrutty
| #
darknet market links https://github.com/aresmarketlink0ru72/aresmarketlink – dark web markets
Diplomi_fvol
| #
купить диплом высшее дистанционно prema-diploms.ru .
Mazraoc
| #
Мы можем предложить дипломы психологов, юристов, экономистов и любых других профессий по приятным ценам. Стараемся поддерживать для покупателей адекватную политику тарифов. Важно, чтобы документы были доступны для большого количества наших граждан.
Приобретение документа, который подтверждает окончание ВУЗа, – это рациональное решение. Заказать диплом любого университета: tutdiploms.com
1win_dhSr
| #
1win войти 1win войти .
Markcen
| #
Покупка квартиры без рисков? Узнайте подробности https://an-rusdom.ru/
Roberturirl
| #
sex cam live
Rabyitabe
| #
dark web marketplaces https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web drug marketplace
1win_rfEr
| #
1вин 1вин .
Donaldlaf
| #
dark web market https://github.com/darknetmarketlinks2025/darknetmarkets – darknet drug store
Sazrwwr
| #
Мы изготавливаем дипломы любых профессий по доступным тарифам.– http://www.tvniederbueren.ch/tvn/wp/?p=2624#comment-372780
Harrymoutt
| #
Онлайнказино Бонсай https://bonsai-casino2.com игровые автоматы, рулетка, покер и живые дилеры! Получайте бонусы за регистрацию, участвуйте в турнирах и выводите выигрыши без задержек. Надёжность, безопасность и азарт – всё в одном месте!
evakyatori spb_xwoa
| #
эвакуатор спб недорого https://www.evakuatormax.ru .
Pingrutty
| #
dark markets 2025 https://github.com/aresmarketlink0ru72/aresmarketlink – darknet drug links
Kennethbob
| #
Актуальні новини будівництва https://dverikupe.com.ua та нерухомості в Україні. Огляди ринку, тренди, технології, законодавчі зміни та експертні думки – все про будівельну галузь на одному сайті!
Toliknaf
| #
darknet markets 2025 https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web marketplaces
Roberturirl
| #
free gay chaturbate
Irvingten
| #
Дізнавайтеся про останні новини https://ampdrive.info електромобілів, супекарів та актуальні події автомобільного світу в Україні. Ексклюзивні матеріали, фото та аналітика для справжніх автолюбителів на AmpDrive.?
DonDonfaita
| #
dark markets 2025 https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet markets
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://bti-nsk.ru/
TCLcaree
| #
Заполните форму заявки на сайте TCL Repair Center или свяжитесь с нами для уточнения деталей. Мы позаботимся о вашей технике TCL и быстро вернем её в строй! Не упустите возможность воспользоваться скидкой до 25% при записи онлайн.
ссылка
Roberturirl
| #
sex cam live
Rabyitabe
| #
darknet market lists https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet markets onion
Donaldlaf
| #
dark market onion https://github.com/darknetmarketlinks2025/darknetmarkets – darknet market links
Pingrutty
| #
dark web marketplaces https://github.com/aresmarketlink0ru72/aresmarketlink – dark web market links
lkjhCype
| #
https://spbzn.ru/crm/pgs/?filmu_zagadki__magiya_tainstvennogo_kino.html нечто плохое дурное 3 буквы
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://crystalit34.ru/
PhillipNaf
| #
селезинка
Toliknaf
| #
darkmarket 2025 https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet websites
Roberturirl
| #
gay daddy cams
DonDonfaita
| #
dark web sites https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet site
GeorgeTom
| #
Source:
http://stroidom-blog.ru
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://cspkaliningrad.ru/
Roberturirl
| #
live sex cams xxx
JamieFam
| #
лідії іменини
Pingrutty
| #
darknet markets onion https://github.com/aresmarketlink0ru72/aresmarketlink – darknet websites
Rabyitabe
| #
darknet drugs https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark market 2025
Donaldlaf
| #
dark web sites https://github.com/darknetmarketlinks2025/darknetmarkets – darkmarket url
1win_anKi
| #
1winn https://1win10.am/ .
JamieFam
| #
іменини ольги за церковним календарем
Sazrubt
| #
купить диплом с занесением в реестр в нижнем тагиле
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://dachnoe-house.ru/
Toliknaf
| #
dark web drug marketplace https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet websites
Roberturirl
| #
gay nude webcam
DonDonfaita
| #
darkmarket 2025 https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet markets links
Terencesam
| #
Top casino с выводом фриспины без депозита
Pingrutty
| #
dark market https://github.com/aresmarketlink0ru72/aresmarketlink – dark market 2025
ремонт техники в мск
| #
Профессиональный сервисный центр по ремонту бытовой техники с выездом на дом.
Мы предлагаем:ремонт бытовой техники в мск
Наши мастера оперативно устранят неисправности вашего устройства в сервисе или с выездом на дом!
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://fasad-alex.ru/
ThomasBor
| #
https://komiinform.ru/nt/7702
Rabyitabe
| #
darknet markets onion address https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet drug links
Donaldlaf
| #
onion dark website https://github.com/darknetmarketlinks2025/darknetmarkets – dark web drug marketplace
remontcaree
| #
Ремонт тепловизоров Pulsar в Москве: LRF XP50, XD50S, XD19S, XD38S, Lite XQ30V, XQ50F, XQ38F, XP38, XP28, Merger LRF XT50, Merger LRF XP50, Axion 2 LRF, Axion XM30F, Axion XQ30 PRO. Закажите звонок для бесплатной диагностики тепловизора pulsar подробнее
Toliknaf
| #
dark market onion https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark market link
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://fisher-kp.ru/
FrankFiP
| #
https://wessta.ru
DonDonfaita
| #
dark market link https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark market link
Pingrutty
| #
darknet sites https://github.com/aresmarketlink0ru72/aresmarketlink – darknet links
Rabyitabe
| #
dark web drug marketplace https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web market links
Donaldlaf
| #
darknet drugs https://github.com/darknetmarketlinks2025/darknetmarkets – darknet markets url
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://fortuna-i-k.ru/
Pingrutty
| #
darknet site https://github.com/aresmarketlink0ru72/aresmarketlink – darknet markets links
PhillipNaf
| #
червона риба під шубою
Toliknaf
| #
darknet websites https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darkmarket url
Waynehem
| #
Актуальні новини політики https://insideukr.com в Україні та світі. Аналітика, думки експертів, головні події дня та ексклюзивні матеріали – будьте в курсі разом із нами!
WilliePar
| #
Honey Money Казино https://honeymoneycasino.ru это захватывающий мир азартных игр с щедрыми бонусами, быстрыми выплатами и огромным выбором слотов, рулетки, покера и других развлечений. Играй онлайн в любое время, участвуй в турнирах и получай эксклюзивные награды.
DonDonfaita
| #
dark market url https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet site
AnthonyBit
| #
Лучшие онлайн казино booi промокод рейтинг топовых игровых платформ с лицензией, щедрыми бонусами, быстрыми выплатами и широким выбором слотов, покера, рулетки и других азартных игр. Мы собрали проверенные казино с высоким рейтингом, безопасностью и выгодными акциями.
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://hertz-lumen.ru/
Rabyitabe
| #
darknet links https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet markets onion
Donaldlaf
| #
best darknet markets https://github.com/darknetmarketlinks2025/darknetmarkets – darknet markets links
Pingrutty
| #
dark web drug marketplace https://github.com/aresmarketlink0ru72/aresmarketlink – darknet markets onion
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://homedecorsochi.ru/
Toliknaf
| #
darknet market [url=https://github.com/darknetmarketlistv8tg0/darknetmarketlist ]darknet websites [/url]
DonDonfaita
| #
darknet market https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark market list
Brianmon
| #
керамогранит 60×120 https://keramogranit213.ru
ShawnWhoks
| #
плитка керамическая настенная купить https://magazin-keramogranit.ru
Larryheive
| #
Купить телевизоры на дачу https://televizory-dlya-dachi.ru в интернет-магазине по низкой цене! При покупке дачных телевизоров на сайте воспользуйтесь скидками, акциями, бонусной программой.
1win casino mexico_evMa
| #
sitio oficial de 1win 1win7.com.mx .
1win_gfPl
| #
1win kg 1win kg .
1win_rama
| #
1 win pro http://www.1win16.com.kg .
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://instrument75.ru/
Pingrutty
| #
darkmarket list https://github.com/aresmarketlink0ru72/aresmarketlink – dark web link
Dnrtcce
| #
Где купить диплом по актуальной специальности?
Мы изготавливаем дипломы любой профессии по доступным ценам. Мы предлагаем документы техникумов, которые расположены в любом регионе России. Вы имеете возможность заказать качественно напечатанный диплом за любой год, в том числе документы старого образца СССР. Документы выпускаются на “правильной” бумаге высшего качества. Это дает возможности делать государственные дипломы, не отличимые от оригинала. Они заверяются необходимыми печатями и подписями. Стараемся поддерживать для заказчиков адекватную политику цен. Для нас очень важно, чтобы дипломы были доступными для большинства наших граждан. diplom-profi.ru/kupit-diplom-v-bryanske-2-4
Rabyitabe
| #
darknet markets https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark websites
Donaldlaf
| #
darknet market list https://github.com/darknetmarketlinks2025/darknetmarkets – darknet market
Terencesam
| #
Бонусы без отыгрыша за регистрацию Игровые автоматы лучшие
Toliknaf
| #
darkmarket list https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web marketplaces
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://integral-sb.ru/
DonDonfaita
| #
darknet market links https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – best darknet markets
GeorgeTom
| #
Source:
http://stroidom-blog.ru
Pingrutty
| #
darknet drug store https://github.com/aresmarketlink0ru72/aresmarketlink – darkmarket list
Sazraem
| #
Мы можем предложить дипломы любой профессии по приятным тарифам. Цена зависит от определенной специальности, года выпуска и ВУЗа. Всегда стараемся поддерживать для клиентов адекватную политику тарифов. Важно, чтобы дипломы были доступными для большинства граждан. диплом с внесением в реестр
lkjhCype
| #
https://ecotruck.su/images/pgs/iz_rossii_s_lubovu__kino_90_h.html фильм где семья перевозила траву в фургоне
SEO PBN
| #
Thankyou, I will surround you
Rabyitabe
| #
darkmarket 2025 https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet links
Donaldlaf
| #
darkmarket link https://github.com/darknetmarketlinks2025/darknetmarkets – dark market list
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://kapplus.ru/
remontcaree
| #
Нужен качественный и оперативный ремонт тепловизоров Guide в Москве? Сервисный центр предлагает профессиональную помощь, доступную каждому владельцу термографических устройств бренда Guide. Мы устраняем все типы неисправностей, гарантируя высокое качество услуг и оперативные сроки работы.подробнее
WillieSarce
| #
Натяжные потолки с установкой https://natyazhnye-potolki2.ru стильное и практичное решение для любого интерьера. Предлагаем широкий выбор фактур и цветов, качественные материалы, быстрый монтаж и гарантию на работу. Устанавливаем потолки любой сложности в квартирах, домах, офисах.
Stephenmycle
| #
Отдых в Анапе https://otdyh-vanape.ru идеальный выбор для всей семьи! Чистые песчаные пляжи, теплое море, развитая инфраструктура и развлечения на любой вкус. Гостиницы, отели, частный сектор – найдите идеальное жилье.
1win_mfOn
| #
сайт 1win официальный сайт вход сайт 1win официальный сайт вход .
1win_orKa
| #
игра ракета на деньги 1win http://1win103.com.kg/ .
Toliknaf
| #
darknet markets links https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web markets
Jamesbluem
| #
Музыкальный проект “Дягилев”
DonDonfaita
| #
darknet markets url https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web market links
Pingrutty
| #
dark web link https://github.com/aresmarketlink0ru72/aresmarketlink – darknet drugs
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://kp-novoe-sonino.ru/
DanielExons
| #
хороший недорогой телевизор 43 недорогие телевизоры 43 дюйма
WilliamRon
| #
https://sqlmaxipro.pro/
Trefwpl
| #
Купить документ института можно у нас в Москве. diplomk-vo-vladivostoke.ru/kupit-shkolnij-attestat-2-2
Lazrprm
| #
Где заказать диплом специалиста?
Купить диплом ВУЗа по доступной стоимости вы можете, обращаясь к проверенной специализированной фирме.: sdiplom.ru
WilliamDix
| #
Мы изготавливаем дипломы любых профессий по приятным тарифам. Дипломы производятся на фирменных бланках Приобрести диплом об образовании diplomt-tver69.ru
Rabyitabe
| #
dark web markets https://github.com/tormarkets2025ukaz1/tormarkets2025 – bitcoin dark web
Donaldlaf
| #
darknet markets links https://github.com/darknetmarketlinks2025/darknetmarkets – dark web markets
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://kvartiranahodynke.ru/
1win_rdsl
| #
1 win официальный сайт 1win17.com.kg .
Pingrutty
| #
dark market url https://github.com/aresmarketlink0ru72/aresmarketlink – darknet websites
Toliknaf
| #
darknet drug market https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet links
Xazriuy
| #
Купить диплом ВУЗа!
Мы изготавливаем дипломы любых профессий по выгодным ценам. Вы покупаете документ в надежной и проверенной временем компании. : diplom-zentr.com/kupit-diplom-gumf-o-visshem-obrazovanii-na-blanke-goznak/
DonDonfaita
| #
darknet drug market https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web market urls
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://lume42.ru/
Life Experience Degree
| #
The wrongful convictions of hundreds of sub-postmasters and sub-postmistresses in the recent Post Office scandal demonstrates what can happen when the victim also plays the role of prosecutor, as happens in TV licensing cases.
https://www.oneidauniversity.com/
Life Experience Degrees
| #
Fantastic beat ! I would like to apprentice whilst you amend your site, how can i subscribe for a weblog web site? The account aided me a appropriate deal. I had been tiny bit acquainted of this your broadcast provided vibrant clear idea.
https://www.charlestonstateuniversity.com/
Danielbor
| #
Все о компьютерных играх https://lifeforgame.ru обзоры новых проектов, рейтинги, детальные гайды, новости индустрии, анонсы и системные требования. Разбираем особенности геймплея, помогаем с настройками и прохождением. Следите за игровыми трендами, изучайте секреты и погружайтесь в мир гейминга.
lkjhCype
| #
https://lepidopterolog.ru/media/pgs/kinovselennaya_marvel__istoriya__filmu_i_vliyanie_na_pop_kulturu.html триггер все сезоны смотреть онлайн бесплатно в хорошем качестве
JessieEnlib
| #
телевизор 32 smart tv m1 32 https://televizory-smart-tv.ru
Eanrxwo
| #
Для эффективного продвижения вверх по карьерной лестнице требуется наличие официального диплома о высшем образовании. Быстро купить диплом университета у проверенной фирмы: diplom-club.com/kupit-diplom-v-rostove-na-donu-10/
WilliamGoorm
| #
телевизор lg oled 65 oled телевизор обзор
Rabyitabe
| #
darknet markets https://github.com/tormarkets2025ukaz1/tormarkets2025 – best darknet markets
Donaldlaf
| #
darknet links https://github.com/darknetmarketlinks2025/darknetmarkets – darknet drug links
1win_hnEn
| #
что делать с бонусным балансом на 1win https://1win111.com.kg/ .
Pingrutty
| #
dark websites https://github.com/aresmarketlink0ru72/aresmarketlink – bitcoin dark web
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://mastera-taganroga.ru/
Toliknaf
| #
dark markets https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web drug marketplace
opticcaree
| #
Ремонт оптических и коллиматорных прицелов Sightmark перейти
DonDonfaita
| #
dark market https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web market urls
ThomasBor
| #
Dota2
Pingrutty
| #
dark market 2025 https://github.com/aresmarketlink0ru72/aresmarketlink – dark markets 2025
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://metallservis38.ru/
Rabyitabe
| #
dark web market list https://github.com/tormarkets2025ukaz1/tormarkets2025 – darkmarket url
ThomasVep
| #
попробуйте эту доставку купить цветы всегда свежие цветочки, быстрая и бесплатная доставка, сервис огонь!
Donaldlaf
| #
dark web market links https://github.com/darknetmarketlinks2025/darknetmarkets – darkmarket
FrankFiP
| #
https://holodokdv.ru
Toliknaf
| #
dark market onion https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web market urls
Jariorghr
| #
Добрый день!
Руководители серьезных предприятий довольно часто выбирают соискателей, окончивших университет. Особенно в приоритете престижные учебные заведения. Однако учиться целых пять лет – это дорого, далеко не у всех есть такая возможность. Приобрести документ – оптимальный выход.
Бывают и непредвиденные случаи, когда диплом утерян. Далеко не всегда возможно оперативно и без осложнений восстановить его, особенно если университет закрыт или располагается в другом регионе России. Бюрократия отнимает массу времени и нервов.
Для максимально быстрого продвижения вверх по карьерной лестнице потребуется наличие официального диплома института. Но зачастую в жизни случается так, что сложные обстоятельства не позволяют с успехом окончить учебу, получив важный документ.
Купить диплом о высшем образовании
Наши специалисты предлагают выгодно купить диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, штампами, подписями должностных лиц. Диплом пройдет любые проверки, даже с использованием специфических приборов. Решайте свои задачи максимально быстро с нашей компанией.
Где купить диплом специалиста? rdiplomix.com/
Iariorzgj
| #
Купить документ о получении высшего образования вы имеете возможность в нашем сервисе. misplacedxsheath.dreamwidth.org/750.html?mode=reply
DonDonfaita
| #
darknet site https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web link
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://mielramenki.ru/
Sazrmui
| #
Студенческая жизнь прекрасна, пока не приходит время писать диплом, как это случилось со мной. Не стоит отчаиваться, ведь существуют компании, которые помогают с написанием и защитой диплома на высокие оценки!
Сначала я искал информацию по теме: купить диплом колледжа цена, диплом медсестры с занесением в реестр спб, купить дипломы колледжа, купить официальный диплом о среднем специальном образовании, купить диплом пту с занесением в реестр, а потом наткнулся на diplomybox.com/kupit-nastoyashhij-diplom
Pingrutty
| #
dark web market urls https://github.com/aresmarketlink0ru72/aresmarketlink – darknet drug store
RonaldToulp
| #
Опытные разработчики Битрикс помогут перенести ваш бизнес в онлайн, все условия сотрудничества по ссылке https://addons.mozilla.org/ru/firefox/user/18650346/
Rabyitabe
| #
dark web market list https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark websites
Donaldlaf
| #
dark web market links https://github.com/darknetmarketlinks2025/darknetmarkets – dark market link
RonaldToulp
| #
Собственное производство наградной продукции для любых мероприятий. Ассортимент по ссылке https://roomstyler.com/users/nagradion
Markcen
| #
Хотите сдать квартиру в аренду? Читайте проверенные советы https://moderntechnology22.ru/
lkjhCype
| #
http://kuzovkirov.ru/wp-content/pgs/romanticheskie_komedii__spisok_luchshih_smeshnuh_filmov_o_lubvi.html канал мультимания программа передач на сегодня москва
Toliknaf
| #
dark web marketplaces https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web markets
DonDonfaita
| #
dark market list https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web sites
BillyShone
| #
Все о недвижимости https://ks-inginiring.ru покупка, аренда, ипотека. Разбираем рыночные тренды, юридические тонкости, лайфхаки для выгодных сделок. Помогаем выбрать квартиру, рассчитать ипотеку, проверить документы и избежать ошибок при сделках с жильем. Актуальные статьи для покупателей, арендаторов и инвесторов.
DavidWog
| #
Все о недвижимости https://brigantina-stroy.ru покупка, аренда, ипотека. Разбираем рыночные тренды, юридические тонкости, лайфхаки для выгодных сделок. Помогаем выбрать квартиру, рассчитать ипотеку, проверить документы и избежать ошибок при сделках с жильем. Актуальные статьи для покупателей, арендаторов и инвесторов.
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://monolitrealestate.ru/
Stevenson
| #
https://vavadaonc10.fun/
Donaldlaf
| #
darknet market links https://github.com/darknetmarketlinks2025/darknetmarkets – darknet drug links
eskort_swma
| #
эскорт услуги москва эскорт услуги москва .
Pingrutty
| #
dark market https://github.com/aresmarketlink0ru72/aresmarketlink – dark market url
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://morozova26.com/
Toliknaf
| #
darknet marketplace https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet websites
DonDonfaita
| #
darknet drug store https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet links
GeorgeTom
| #
Source:
http://ic51.ru
DavidNes
| #
Все о компьютерных играх https://lifeforgame.ru/ обзоры новых проектов, рейтинги, детальные гайды, новости индустрии, анонсы и системные требования. Разбираем особенности геймплея, помогаем с настройками и прохождением. Следите за игровыми трендами, изучайте секреты и погружайтесь в мир гейминга.
Rabyitabe
| #
darknet drug links https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet market list
Markcen
| #
Хотите сдать квартиру в аренду? Читайте проверенные советы https://new-fstore.ru/
Toliknaf
| #
darkmarket url https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darkmarkets
DonDonfaita
| #
darkmarket 2025 https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet markets onion
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://okkord.ru/
Pingrutty
| #
dark web market https://github.com/aresmarketlink0ru72/aresmarketlink – dark market
Rabyitabe
| #
dark markets 2025 https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web market list
DavidWog
| #
Все о недвижимости https://knyaz-dolgoruky.ru покупка, аренда, ипотека. Разбираем рыночные тренды, юридические тонкости, лайфхаки для выгодных сделок. Помогаем выбрать квартиру, рассчитать ипотеку, проверить документы и избежать ошибок при сделках с жильем. Актуальные статьи для покупателей, арендаторов и инвесторов.
opendance
| #
зумба тренировка научиться шаффл
sharedsteam
| #
Looking for free steam account? We regularly share working accounts with games, bonuses, and tips. Subscribe now and don’t miss new giveaways! Only verified and active accounts.
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://ooo-sk-micom.ru/
lkjhCype
| #
https://naturalbeekeeping.ru/wp-content/pgs/filmu_140.html список фильмов которые должен посмотреть каждый
Herbertargum
| #
кофемашина philips
Toliknaf
| #
darknet markets https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darkmarket link
DonDonfaita
| #
darkmarket link https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet links
Pingrutty
| #
dark market 2025 https://github.com/aresmarketlink0ru72/aresmarketlink – dark market onion
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://privateconstruction.ru/
Donaldlaf
| #
dark market link https://github.com/darknetmarketlinks2025/darknetmarkets – dark web drug marketplace
Rabyitabe
| #
best darknet markets https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web marketplaces
servicecaree
| #
Кофемашины Jura — это эталоны надежности, технологичности и дизайна. Сервисный центр Jura Repair Center предлагает профессиональный ремонт кофемашин Jura, чтобы ваша техника снова радовала вас безупречным кофе. подробнее
взять микрозайм без отказа на карту
| #
Срочная новость из мира финансов! Известный экономист раскрыл секрет займа всем без отказа. Проверенные МФО с гослицензией выдают до 30 000 рублей под 0.8% в день. Главное преимущество – официальное оформление и защита от коллекторов.
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://prudmarket.ru/
Toliknaf
| #
darknet markets links https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darkmarket 2025
Pingrutty
| #
dark market list https://github.com/aresmarketlink0ru72/aresmarketlink – darkmarket 2025
DonDonfaita
| #
dark web drug marketplace https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web link
Diplomi_kxer
| #
купить диплом 2015 купить диплом 2015 .
Rabyitabe
| #
darkmarket url https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet marketplace
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://pultmaster-pro.ru/
MetaMask Download
| #
MetaMask Extension is a game-changer! It allows me to interact with dApps without compromising security. Highly recommend it!
Terencesam
| #
бонусы за регистрацию Бонусы без отыгрыша за регистрацию
Pingrutty
| #
darkmarket list https://github.com/aresmarketlink0ru72/aresmarketlink – darkmarket
Toliknaf
| #
dark market onion https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark markets 2025
ThomasKef
| #
компот рецепт Eda321 – ваш незаменимый помощник в мире кулинарии! Мы собрали для вас богатую коллекцию рецептов, способных удовлетворить любой вкус и уровень подготовки. Откройте для себя простые, вкусные и быстрые рецепты, идеально подходящие для приготовления блюд на каждый день. Наша домашняя кухня – это кладезь идей для тех, кто ценит традиционные русские блюда. Начинающим кулинарам мы предлагаем рецепты с подробными фото, которые помогут освоить азы кулинарного искусства. Опытные кулинары найдут для себя интересные и оригинальные блюда из мяса, рыбы, курицы и картофеля. На Eda321 вы всегда найдете вдохновение для кулинарных экспериментов и сможете порадовать своих близких вкусными и полезными блюдами. Присоединяйтесь к нам, и вместе мы откроем новые горизонты в мире кулинарии!
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://remont-nvr.ru/
DavidWog
| #
Все о недвижимости https://magdesi.ru покупка, аренда, ипотека. Разбираем рыночные тренды, юридические тонкости, лайфхаки для выгодных сделок. Помогаем выбрать квартиру, рассчитать ипотеку, проверить документы и избежать ошибок при сделках с жильем. Актуальные статьи для покупателей, арендаторов и инвесторов.
Davidnip
| #
телевизоры samsung qled 4k https://televizory-qled.ru
RobertRon
| #
Все о компьютерных играх lifeforgame.ru обзоры новых проектов, рейтинги, детальные гайды, новости индустрии, анонсы и системные требования. Разбираем особенности геймплея, помогаем с настройками и прохождением. Следите за игровыми трендами, изучайте секреты и погружайтесь в мир гейминга.
FrankFiP
| #
https://anrespectplus.ru
Rabyitabe
| #
darkmarket link https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet sites
Pingrutty
| #
darknet market list https://github.com/aresmarketlink0ru72/aresmarketlink – dark market list
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://repinskieozera.ru/
Toliknaf
| #
dark web market urls https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet links
DonDonfaita
| #
dark web markets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darkmarkets
1win md_pooi
| #
1win casino http://www.1win6.md/ .
1win md_tgkn
| #
1win.com официальный сайт https://1win7.md .
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://sanrival.ru/
Duncanmor
| #
можно ли отправить посылку в россию Бесплатные путешествия — реальность! Я расскажу как без затрат посещать теплые страны. Многие россияне обосновались на Бали, и им часто требуется доставка вещей из России. За транспортировку они готовы платить от 20 долларов за килограмм или фиксированную сумму за посылку. Берем два чемодана, заполняем их посылками, и вот мы уже на Бали, да еще и с прибылью!
Rabyitabe
| #
darknet markets links https://github.com/tormarkets2025ukaz1/tormarkets2025 – dark web market urls
Donaldlaf
| #
darknet market lists https://github.com/darknetmarketlinks2025/darknetmarkets – darknet drug store
Pingrutty
| #
dark web market list https://github.com/aresmarketlink0ru72/aresmarketlink – dark web market urls
GeorgeTom
| #
Source:
– http://ic51.ru
servicecaree
| #
Почему не работает робот-пылесос: ТОП 8 самых расспространенных причин, которые мы сможем решить самостоятельно. здесь
Times Startup
| #
Cek my web blog rn
Herbertargum
| #
холодильники в молдове
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные статьи и советы — https://servit74.ru/
Toliknaf
| #
dark websites https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet markets 2025
DonDonfaita
| #
darknet markets url https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark market onion
Pingrutty
| #
darknet links https://github.com/aresmarketlink0ru72/aresmarketlink – dark web market list
Donaldlaf
| #
dark web sites https://github.com/darknetmarketlinks2025/darknetmarkets – darknet websites
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://shatimtrade-kizlyar.ru/
lkjhCype
| #
https://astorplace.ru/wp-content/pgs/filmu_onlayn___novuy_uroven_domashnego_kinoteatra.html киноафиша спб плаза жемчужная сегодня
Toliknaf
| #
darknet markets url https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web market urls
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://sibotdelka.ru/
1win_kber
| #
1win онлайн http://1win11.am .
Pingrutty
| #
best darknet markets https://github.com/aresmarketlink0ru72/aresmarketlink – darknet sites
DonDonfaita
| #
darknet market links https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web drug marketplace
1win_ilSt
| #
1win armenia https://1win13.am/ .
1win_ebot
| #
1вин официальный регистрация 1win5.am .
lifeforgameru
| #
Мир компьютерных игр http://lifeforgame.ru Мы расскажем о лучших новинках, секретах прохождения, системных требованиях и игровых трендах. Новости, гайды, обзоры и рейтинги – всё, что нужно геймерам.
JeremyGew
| #
Покупка, аренда, ипотека https://geodizond.ru всё о недвижимости в одном блоге! Советы по выбору жилья, юридические аспекты, анализ цен и прогнозы рынка. Рассказываем, как грамотно оформить ипотеку, проверить документы и избежать ошибок при сделках с недвижимостью. Будьте в курсе всех изменений и трендов!
Donaldlaf
| #
darknet marketplace https://github.com/darknetmarketlinks2025/darknetmarkets – darknet links
AnthonyJuink
| #
уроки шаффла школа танцев
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://snegirieko.com/
Pingrutty
| #
darknet drugs https://github.com/aresmarketlink0ru72/aresmarketlink – darkmarket link
Toliknaf
| #
darknet marketplace https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark web market urls
phoenixautobodyshopmailm
| #
Unlock your vehicle’s potential with our top-notch auto tuning services! Unleash your ride into a stunning masterpiece with our expert team. We specialize in modifications that cater to your unique style. Our exceptional solutions ensure optimal performance and eye-catching design . Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://americasbestcertifiedautobody.com/ today and take the first step towards your dream car!
DonDonfaita
| #
bitcoin dark web https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web marketplaces
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://so35.ru/
FrankFiP
| #
https://химчисткамебели.online
Rabyitabe
| #
darkmarket https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet market links
Pingrutty
| #
darknet markets 2025 https://github.com/aresmarketlink0ru72/aresmarketlink – darkmarkets
Stevenson
| #
https://vavada37.com/
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://sochi-park-dom.ru/
Terencesam
| #
1xslots бездепозитный бонус Играть игровые автоматы на деньги
1win_jlpr
| #
1win.am http://1win12.am/ .
Toliknaf
| #
darknet websites https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darkmarket 2025
LarryThope
| #
купить волосы в спб Купить волосы СПБ! В Санкт-Петербурге: новый уровень вашего стиля! Хотите обновить свой образ и добавить яркие акценты? Приобретение волос в СПб — это отличное решение для вас! Мы предлагаем широкий ассортимент натуральных волос, идеально подходящих для наращивания, создания причесок и стильных аксессуаров.
DonDonfaita
| #
darkmarkets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark websites
JamesAlirl
| #
Играйте в онлайн-казино https://t.me/casino_na_ruble/11 с рублевыми счетами! Надежные казино с моментальными выплатами, бонусами и фриспинами. Пополнение через карты, кошельки и криптовалюту. Выбирайте топовые игры и выигрывайте без лишних комиссий!
Billybes
| #
Онлайн казино игровые автоматы играть бесплатно с лучшими бонусами и шансом на крупный выигрыш! Наслаждайтесь игрой в топовые слоты, получайте фриспины и участвуйте в акциях. Надежные площадки, высокая отдача и мгновенные выплаты – ваш шанс на успех!
JeffreyUnimb
| #
Играйте в онлайн-казино https://t.me/casino_bonus_bezdep/11 топовые игровые автоматы, лайв-дилеры, мгновенные выплаты и бонусы для новых игроков. Наслаждайтесь честной игрой, удобными платежными методами и крупными выигрышами!
Galenboows
| #
эскорт услуги
модели эскорт
Rabyitabe
| #
darknet markets https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet markets onion address
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные статьи и советы — https://technovoda.ru/
Pingrutty
| #
dark web marketplaces https://github.com/aresmarketlink0ru72/aresmarketlink – darknet links
Georgeuncog
| #
баня цена
lkjhCype
| #
https://www.urank.ru/pgs/dlinnue_serialu___pochemu_mu_ih_tak_lubim_.html синема 5 энгельс облака афиша на сегодня
servicecaree
| #
Ремонт серверов HP и другой электроники HP в Москве – Сервисный центр HP здесь
Toliknaf
| #
best darknet markets https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet markets
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://territoria-we-stars.ru/
DonDonfaita
| #
darknet drug store https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark web link
SergioGaf
| #
волосы для наращивания Волосы для наращивания – это не просто тренд, это возможность мгновенно преобразиться, добавить объема и длины, о которой вы всегда мечтали. Существует множество техник: от горячего и холодного наращивания до ленточного и микрокапсульного, каждая из которых имеет свои преимущества и подходит для разных типов волос.
Pingrutty
| #
darkmarket list https://github.com/aresmarketlink0ru72/aresmarketlink – darknet market links
Stevenson
| #
vavada jogo
sohbet
| #
great blog very nice articles I will follow you continuously sohbet odaları
Rabyitabe
| #
darkmarket 2025 https://github.com/tormarkets2025ukaz1/tormarkets2025 – darkmarket 2025
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://texnotect.ru/
Toliknaf
| #
darknet market https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark market list
Pingrutty
| #
darknet site https://github.com/aresmarketlink0ru72/aresmarketlink – dark market 2025
DonDonfaita
| #
dark web markets https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark market list
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://tolmi74.ru/
Galenboows
| #
вип эскорт агентство
эскорт москва цены
1win_uwei
| #
1winn http://www.1win6.am .
1win_lksn
| #
баланс 1win https://www.1win7.am .
Rabyitabe
| #
dark market url https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet drug links
Samsungcaree
| #
На телефоне Samsung некорректно работает сенсорный экран: 8 причин и методов исправить самостоятельно перейти
Sazrbsc
| #
купить бланк аттестат
Galenboows
| #
сайт эскорт услуг
вип эскорт
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://topkadastr-crimea.ru/
Pingrutty
| #
darknet sites https://github.com/aresmarketlink0ru72/aresmarketlink – darknet drug links
Toliknaf
| #
darkmarket https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet links
1win_vnPn
| #
1 win mexico 1 win mexico .
DonDonfaita
| #
darknet site https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet markets
1win_vdei
| #
1win casino mexico http://1win5.com.mx/ .
Donaldlaf
| #
darknet market list https://github.com/darknetmarketlinks2025/darknetmarkets – dark web market list
lkjhCype
| #
https://histor-ru.ru/wp-content/pgs/serialu_pro_turmu___zahvatuvaushiy_mir_za_resh_tkoy.html смотреть онлайн фильмы 2024 года бесплатно в хорошем качестве новинки
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://uk-arbat.ru/
Galenboows
| #
элитный эскорт
эскорт агенство
Pingrutty
| #
darknet market https://github.com/aresmarketlink0ru72/aresmarketlink – dark web markets
Andrewmic
| #
Glory Casino
Toliknaf
| #
dark websites https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet markets onion address
Andrewmic
| #
Glory Casino
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://vega-snab.ru/
DonDonfaita
| #
dark web sites https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet markets onion
GoogleMapscaree
| #
Где отремонтировать сложную оптику: тепловизоры, прицелы, дальномеры, коллиматоры, монокуляры. Вот на гугл картках отметка сервисного центра карта
AubreyReoft
| #
The best casino https://t.me/new_retro_casino_site/14! Classic slot machines, generous bonuses and reliable payouts. Enjoy the excitement of retro slots and play on a proven platform!
Donaldlaf
| #
dark web link https://github.com/darknetmarketlinks2025/darknetmarkets – dark market list
Rabyitabe
| #
darknet markets links https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet links
VictorCaf
| #
Организация офисных переездов под ключ с грузчиками и специализированным транспортом, подробности тут https://www.imdb.com/user/ur189257174/?ref_=nv_usr_prof_2
Pingrutty
| #
darknet drug market https://github.com/aresmarketlink0ru72/aresmarketlink – darknet site
Raymondnus
| #
Все, что вам нужно для игры https://t.me/vavada_casino_vhod/11! Игровые автоматы, рулетка, покер, живые дилеры и эксклюзивные бонусы. Наслаждайтесь качественной игрой с мгновенными выплатами и надежными провайдерами!
phoenixautobodyshopmailm
| #
Unlock your vehicle’s potential with our top-notch auto tuning services! Enhance your ride into a stunning masterpiece with our expert team. We specialize in refinements that cater to your unique style. Our high-quality solutions ensure optimal performance and stylish look . Don’t settle for average; elevate your driving experience and turn heads on the road! Visit us at https://americasbestcertifiedautobody.com/ today and take the first step towards your dream car!
WilliamdremI
| #
Играйте онлайн в JVSpin Казино – лицензионное онлайн-казино с лучшими слотами, лайв-играми и щедрыми бонусами. Пополняйте счет удобными способами, получайте награды и выигрывайте реальные деньги!
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://vernadskogo-loft.ru/
VictorCaf
| #
Инженерные решения для безопасной прокладки кабелей, подробности тут https://pixai.art/@tmgroup/artworks
Cazrrou
| #
Мы готовы предложить документы любых учебных заведений, расположенных на территории всей РФ. diplomoz-197.com/kupit-diplom-v-novorossijske-4-2
Toliknaf
| #
dark markets https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet market list
RobertWag
| #
кайт школа анапа
DonDonfaita
| #
darknet marketplace https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet markets links
1win_mbsa
| #
1 win casino http://1win4.com.mx .
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://worldstone33.ru/
Pingrutty
| #
dark websites https://github.com/aresmarketlink0ru72/aresmarketlink – darknet markets
Rabyitabe
| #
tor drug market https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet markets 2025
lkjhCype
| #
http://spincasting.ru/core/art/index.php?filmu_pro_biznes__vdohnovenie_i_uroki_dlya_predprinimateley.html телевизор на андроиде 43 дюйма купить
MalcolmWag
| #
Купить солнечные панели
Markcen
| #
Покупка квартиры без рисков? Узнайте подробности https://xn—-7sbajm1bmmamhew.xn--p1ai/
repaircentercaree
| #
Если вам нужна помощь с техникой MSI, вы обратились по адресу. Наш сервисный центр MSI специализируется на ремонте ноутбуков, компьютеров, видеокарт, материнских плат, мониторов, моноблоков и планшетов MSI. Мы используем только оригинальные запчасти и предоставляем долгосрочные гарантии на ремонт. здесь
GeorgeTal
| #
Играйте в https://t.me/casino_jozz/7 популярное онлайн-казино с широким выбором слотов, настольных игр и лайв-дилеров. Выгодные бонусы, удобные платежные системы и моментальные выплаты ждут вас!
Toliknaf
| #
darkmarket link https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet market
Jameszer
| #
Выбирайте https://t.me/booi_casino_site/10 топовое онлайн-казино с выгодными акциями, эксклюзивными играми и лайв-казино. Присоединяйтесь к сообществу игроков и получайте максимальное удовольствие от игры!
Richardwit
| #
A powerful VPN solution https://github.com/ivanti-it/Ivanti-Secure-Access-Client/releases designed to provide secure remote access to corporate networks. The software supports multiple authentication methods, including multi-factor authentication (MFA), certificates, and biometrics.
VictorLop
| #
Сброс лишнего веса, расстройства пищевого поведения, психологическая коррекция, РПП, избыточная масса тела, помощь психолога. лишний вес
Pingrutty
| #
dark markets 2025 https://github.com/aresmarketlink0ru72/aresmarketlink – darknet markets links
DonDonfaita
| #
darknet markets links https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet links
Donaldlaf
| #
dark market link https://github.com/darknetmarketlinks2025/darknetmarkets – dark market onion
Rabyitabe
| #
dark market 2025 https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet site
LarryTub
| #
Свіжі ідеї дизайну https://dverikupe.od.ua інтер’єру, сучасні тренди, поради щодо декору та ремонту. Створюйте затишок та стиль у кожному куточку вашого дому!
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://xn—-7sbccdscdoffrg1cybj3z.xn--p1ai/
GeorgeTom
| #
Source:
http://ic51.ru
Pingrutty
| #
darkmarket link https://github.com/aresmarketlink0ru72/aresmarketlink – darknet marketplace
Toliknaf
| #
dark web market urls https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet drug market
CharlesCam
| #
диодная лазерная эпиляция спб интимная лазерная эпиляция цены
JamesEvork
| #
свіжі новини криптовалют https://cryptonews.v.ua блокчейна та DeFi. Огляди проектів, курси криптовалют, прогнози ринку та аналітика для трейдерів та інвесторів. Слідкуйте за криптомир з нами!
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные рекомендации — https://xn--90aimcoinaikf.xn--p1ai/
Terencesam
| #
bezdep bonus casino
DonDonfaita
| #
darkmarket list https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – tor drug market
FrankFiP
| #
https://ceramodecol.ru
Donaldlaf
| #
darknet marketplace https://github.com/darknetmarketlinks2025/darknetmarkets – darknet markets links
Rabyitabe
| #
dark market 2025 https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet links
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://xn——6cdbaabncc2b3blicg2a2b7axf0dzfui.xn--p1ai/
Pingrutty
| #
darknet markets onion https://github.com/aresmarketlink0ru72/aresmarketlink – darknet market
Toliknaf
| #
darknet drug store https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet market
GregoryGab
| #
типографии полного цикла в спб типография заказать
sohbet
| #
great blog very nice articles I will follow you continuously sohbet odaları
DonDonfaita
| #
darkmarket url https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – dark markets 2025
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://xn—-8sbqbliyb2m.xn--p1ai/
Rabyitabe
| #
darknet market lists https://github.com/tormarkets2025ukaz1/tormarkets2025 – darkmarket url
Donaldlaf
| #
darknet drug links https://github.com/darknetmarketlinks2025/darknetmarkets – darknet markets onion address
Pingrutty
| #
darknet markets onion https://github.com/aresmarketlink0ru72/aresmarketlink – darknet drug links
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://xn--80agflcgbqkedhbfsfqs3v.xn--p1ai/
Toliknaf
| #
onion dark website https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet markets links
1win_hcoi
| #
1win 1win .
1win_mdmt
| #
ваучеры 1win ваучеры 1win .
DonDonfaita
| #
darknet drug market https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darkmarket list
Rabyitabe
| #
dark web drug marketplace https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet markets 2025
Diplomi_tlKt
| #
Заказать диплом о высшем образовании!
Мы можем предложить документы институтов, расположенных на территории всей России.
Основные преимущества наших дипломов:
• используются только настоящие бланки “Гознак”;
• необходимые подписи руководства;
• мокрые печати университета;
• специальные водяные знаки, нити и другие степени защиты;
• безупречное качество оформления – ошибок не будет;
• любая проверка документа.
10000diplomov.ru/kupit-svidetelstvo-o-rozhdenii-6
Pingrutty
| #
darknet markets https://github.com/aresmarketlink0ru72/aresmarketlink – dark web link
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://xn--2-htbyfc.xn--p1ai/
lkjhCype
| #
https://pandorabox.ru/css/pgs/geroi_multserialov_v_kino.html zz top видео лучшие клипы
1win_ihPi
| #
бонусы казино 1вин 1win3003.ru .
demo mahjong
| #
Main slot demo mahjong ways rupiah resmi dari pg soft cuma di demo mahjong naga merah.
Toliknaf
| #
darknet drug market https://github.com/darknetmarketlistv8tg0/darknetmarketlist – darknet drug store
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://xn--61-6kcq2ag4b6e.xn--p1ai/
Pingrutty
| #
darknet market links https://github.com/aresmarketlink0ru72/aresmarketlink – darknet market links
DonDonfaita
| #
dark market link https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet drugs
Davidunmat
| #
palmslots trustpilot
Rabyitabe
| #
dark market list https://github.com/tormarkets2025ukaz1/tormarkets2025 – darknet market list
Donaldlaf
| #
darknet marketplace https://github.com/darknetmarketlinks2025/darknetmarkets – dark web link
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://xn—–7kchcrgop2a1bbbeqft7m5b.xn--p1ai/
Pingrutty
| #
darknet market list https://github.com/aresmarketlink0ru72/aresmarketlink – darknet markets links
Toliknaf
| #
darknet markets onion https://github.com/darknetmarketlistv8tg0/darknetmarketlist – dark market onion
Jariorqyh
| #
Здравствуйте!
Начальники довольно часто выбирают кандидатов, которые закончили ВУЗ. Особенно в приоритете элитные учебные заведения. Но учиться пять лет – это долго и дорого, далеко не у каждого есть такая возможность. Приобрести документ – оптимальный выход.
Могут быть и непредвиденные обстоятельства, когда диплом теряется или портится. Далеко не всегда возможно быстро и без проблем восстановить его, особенно если ВУЗ закрыт или располагается в другом регионе страны. Бюрократия отнимает множество времени.
Для быстрого продвижения вверх по карьере понадобится наличие официального диплома о высшем образовании. Тем не менее часто в жизни случается так, что некоторые трудности мешают благополучно окончить учебу и заполучить желанный документ.
Приобрести диплом ВУЗа
Наша компания предлагает выгодно и быстро приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, штампами, подписями. Документ способен пройти лубую проверку, даже с применением специфических приборов. Решите свои задачи быстро и просто с нашей компанией.
Где приобрести диплом специалиста? rdiplomm24.com/
DonDonfaita
| #
darknet drug market https://github.com/nexusdarkneturlwrf4t/nexusdarkneturl – darknet drug market
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://xn—–7kcoqeea0aaiqm4apq0s.xn--p1ai/
Donaldlaf
| #
darkmarket 2025 https://github.com/darknetmarketlinks2025/darknetmarkets – dark web drug marketplace
GeorgeTom
| #
Source:
http://ic51.ru
1win_numi
| #
1вин партнерка https://1win3004.ru/ .
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://xn—-7sbhyegsibavlngp.xn--p1ai/
Sazrcps
| #
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по невысоким ценам. Преимущества заказа документов в нашем сервисе
Вы приобретаете документ в надежной и проверенной временем компании. Такое решение сэкономит не только денежные средства, но и время.
На этом преимущества не заканчиваются, их гораздо больше:
• Документы изготавливаются на оригинальных бланках с мокрыми печатями и подписями;
• Дипломы любого ВУЗа России;
• Стоимость во много раз меньше той, которую понадобилось бы заплатить на очном и заочном обучении в ВУЗе;
• Удобная доставка в любые регионы Российской Федерации.
Приобрести диплом академии– http://poboltaem.foroactivo.com/post/ – poboltaem.foroactivo.com/post
Pingrutty
| #
darknet market links darknet drug market
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://xn--j1aihe7cc.com/
Toliknaf
| #
dark web market darknet markets
JerryJuism
| #
http://forum.toonboom.ru/index.php?/topic/4809-pr-агентство-в-москве/
Terencesam
| #
Бездепозитные бонусы за регистрацию Бесплатные спины за регистрацию без депозита
DonDonfaita
| #
tor drug market dark market onion
ClaytonShank
| #
Покупка недвижимости и ипотека https://asksteel.ru что нужно знать? Разбираем выбор жилья, условия кредитования, оформление документов и юридические аспекты. Узнайте, как выгодно купить квартиру и избежать ошибок!
Donaldlaf
| #
dark web marketplaces dark web drug marketplace
Pingrutty
| #
darknet links darknet drug market
Trefhax
| #
Приобрести документ о получении высшего образования вы можете у нас в столице. diplomaz-msk.com/kupit-diplom-tolyatti-4
MichaelPak
| #
Hello. It’s my blog https://brilliantcover.ru/.
RobertWag
| #
кайт школа в анапе
Toliknaf
| #
darknet markets links dark market onion
DonDonfaita
| #
darknet markets onion address dark web market list
servicecentercaree
| #
Квадрокоптеры DJI — это высокотехнологичные устройства, обеспечивающие захватывающие возможности в видеосъемке и навигации. Однако даже самые надёжные дроны иногда сталкиваются с проблемами. Эта статья поможет разобраться в частых неисправностях, их причинах, способах устранения и ситуациях, когда без сертифицированного сервисного центра не обойтись. ссылка
Donaldlaf
| #
darknet site dark web markets
Pingrutty
| #
dark market 2025 darknet drugs
VictorCaf
| #
Ваш бизнес заслуживает лучшего — премиальные овощи и зерновые от проверенного поставщика, детали по ссылке https://webyourself.eu/Gordon792
FrankFiP
| #
https://diamantgeo.ru
Toliknaf
| #
darkmarket 2025 dark web market
Pingrutty
| #
darknet markets 2025 darkmarket url
VictorCaf
| #
Ищу работу через этот сайт, быстро и удобно, рекомендую ознакомиться https://www.gamerlaunch.com/community/users/blog/6646995/2364755/job-search-platform/?gid=535
DonDonfaita
| #
dark market onion darkmarket url
Lazraae
| #
Диплом ВУЗа РФ!
Без получения диплома трудно было продвигаться по карьерной лестнице. Именно из-за этого решение о заказе диплома следует считать целесообразным. Заказать диплом о высшем образовании ostome.ru/forumbb/viewtopic.php?f=12&t=274205
Mazrgmt
| #
Привет!
Где заказать диплом специалиста?
Мы изготавливаем дипломы любой профессии по невысоким ценам. Стоимость зависит от выбранной специальности, года получения и образовательного учреждения. Стараемся поддерживать для покупателей адекватную ценовую политику. Для нас важно, чтобы документы были доступны для большинства граждан.
Покупка диплома, который подтверждает обучение в ВУЗе, – это выгодное решение. Попросту подсчитайте, сколько понадобится потратить средств на оплату 5-летнего обучения, на аренду жилья (если учащийся иногородний), на ежедневный проезд до университета и прочие издержки. Выйдет серьезная сумма, которая превышает цены на наши дипломы. А ведь все это время можно уже с успехом работать, занимаясь своей карьерой.
Готовый диплом с приложением целиком и полностью отвечает условиям и стандартам Министерства образования и науки, неотличим от оригинала. Не стоит откладывать свои мечты на потом, реализуйте их с нашей компанией – отправьте заявку на диплом прямо сейчас!
Купить диплом о среднем образовании – запросто! diplomanc.com/
Donaldlaf
| #
darknet market darknet markets onion
1win_tbSl
| #
бк 1вин 1win3005.ru .
Toliknaf
| #
dark market darknet drug market
Pingrutty
| #
tor drug market darkmarket url
Mazrzur
| #
Где купить диплом специалиста?
Мы предлагаем документы об окончании любых университетов РФ. Документы производят на подлинных бланках государственного образца. 39504.org/showthread.php?tid=82709
Taylorrew
| #
ипотека и покупка недвижимости https://luchdom.ru что нужно знать? Разбираем выбор жилья, условия кредитования, оформление документов и юридические аспекты. Узнайте, как выгодно купить квартиру и избежать ошибок!
DonDonfaita
| #
dark web market list darknet markets onion address
Stevereina
| #
Buy elite property in Montenegro: purchase apartments and houses without risks! Current prices, transaction execution, taxes and residence permit. Profitably purchase housing for life, recreation or investment.
Donaldlaf
| #
darknet marketplace darknet markets
servicecaree
| #
Мы знаем, насколько важна для вас надежность техники GoPro, и делаем всё, чтобы вернуть её в рабочее состояние в максимально короткие сроки. Узнайте, почему GoPro Center — лучший выбор для ремонта ваших устройств. Получите бесплатную консультацию по телефону и мы определим стоимость ремонта техники Go Pro. перейти
lkjhCype
| #
https://www.tenox.ru/wp-content/pgs/serialu_pro_shkolu__zahvatuvaushie_istorii__kotorue_stoit_posmotret.html скачать фильм бесплатно на киновасек
Mazrzir
| #
Мы изготавливаем дипломы любой профессии по приятным тарифам. Всегда стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас важно, чтобы дипломы были доступны для большого количества граждан.
Приобретение документа, который подтверждает обучение в университете, – это выгодное решение. Заказать диплом ВУЗа: diplomanrussians.com
Pingrutty
| #
darknet drugs dark web markets
Lazrtfu
| #
Приобрести диплом любого института!
Мы готовы предложить дипломы любой профессии по приятным тарифам— diplom-onlinex.com/kupit-diplom-v-novosibirske-19/
Toliknaf
| #
darknet markets darkmarket link
Mazrsnd
| #
Мы изготавливаем дипломы любых профессий по приятным тарифам. Стараемся поддерживать для покупателей адекватную ценовую политику. Важно, чтобы документы были доступными для большого количества граждан.
Покупка диплома, подтверждающего окончание института, – это рациональное решение. Приобрести диплом университета: diplom-top.ru/kupit-diplomi-o-visshem-3/
DonDonfaita
| #
darknet markets 2025 darkmarket 2025
Jariorywz
| #
Где приобрести диплом специалиста?
Наша компания предлагает выгодно купить диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, штампами, подписями должностных лиц. Наш диплом пройдет любые проверки, даже с применением профессиональных приборов. Достигайте свои цели быстро и просто с нашим сервисом.
Приобрести диплом ВУЗа diplomus-spb.ru/kupit-diplom-s-zaneseniem-v-reestr-11/
Donaldlaf
| #
onion dark website darknet market lists
"https://chrisophia.wiki/index.php/User:ShantellKavanaug"
| #
Подбор авто из Японии с
учетом ваших предпочтений
“https://drapia.org/11-WIKI/index.php/Vttauto_100M”
Pingrutty
| #
darknet markets dark web drug marketplace
Andrewcheex
| #
На площадке представлен широкий ассортимент травматического оружия https://travmat-kupit.ru/
DavidNaw
| #
https://textme.work/
GeorgeTom
| #
Source:
http://ic51.ru
Toliknaf
| #
darkmarket 2025 dark market link
Andrewcheex
| #
На площадке представлен широкий ассортимент травматического оружия https://travmat-kupit.ru/
DonDonfaita
| #
dark web markets dark market onion
"https://clashofcryptos.trade/wiki/User:RudolphChewning"
| #
Доставка авто из Китая в сжатые сроки
“https://wiki.learning4you.org/index.php?title=User:PatrickDecicco”
Donaldlaf
| #
darknet markets 2025 darkmarket 2025
Pingrutty
| #
dark web link bitcoin dark web
servicecaree
| #
Умные часы Garmin являются надежными спутниками спортсменов и активных людей, но даже самые качественные устройства могут столкнуться с проблемами. Одна из наиболее распространенных неисправностей – это сбой в работе зарядки. В этой статье мы рассмотрим основные причины, почему часы Garmin могут перестать заряжаться, и подскажем, как решить проблему. ссылка
Jeffreyslany
| #
Secure your connections https://github.com/aws-ec2/AWS-CLI/releases with FortiClient, ArcGIS Pro, Cisco AnyConnect, and Cisco Secure Client. Reliable VPN clients for remote work, data protection, and corporate network access without threats or information leaks!
"https://talkingheadswiki.com/w/Vttauto_8k"
| #
Как заказать японский авто без лишних
переплат?
“https://www.hohenbergen.de/index.php/Benutzer:WinfredLlanas”
Terencesam
| #
Top casino с выводом Бонусы за регистрацию
Toliknaf
| #
dark markets 2025 dark market url
Pingrutty
| #
darknet markets 2025 darknet sites
JamesEnuts
| #
https://vkontakte.forum.cool/viewtopic.php?id=19599#p53208
DonDonfaita
| #
dark web market list dark market url
Donaldlaf
| #
dark web marketplaces dark web link
Dnrtwtk
| #
Где приобрести диплом специалиста?
Мы изготавливаем дипломы любой профессии по приятным ценам. Мы готовы предложить документы ВУЗов, расположенных на территории всей Российской Федерации. Вы сможете заказать диплом от любого заведения, за любой год, включая сюда документы старого образца. Документы делаются на бумаге высшего качества. Это позволяет делать государственные дипломы, не отличимые от оригинала. Они будут заверены всеми необходимыми печатями и подписями. Стараемся поддерживать для клиентов адекватную ценовую политику. Важно, чтобы документы были доступны для большого количества граждан. diplomius-docs.com/kuplyu-diplom-o-visshem-obrazovanii-2-3
Sazrvhr
| #
Мы изготавливаем дипломы любых профессий по приятным ценам. Цена будет зависеть от выбранной специальности, года выпуска и образовательного учреждения. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Для нас важно, чтобы документы были доступными для большого количества наших граждан. купить диплом о высшем образовании севастополь
Toliknaf
| #
darknet markets 2025 dark market url
Pingrutty
| #
darknet market links dark web link
servicecaree
| #
Сервисный центр Samsung — это надежный партнер для владельцев техники Samsung. Мы предлагаем обширный перечень услуг по ремонту и обслуживанию разнообразных устройств. Скорость, качество и комфорт — три основных принципа нашей работы. перейти
DonDonfaita
| #
darknet markets url tor drug market
Donaldlaf
| #
dark web markets darknet websites
Cazrsdb
| #
Здравствуйте!
Без наличия диплома очень непросто было продвигаться по карьере. В наше время документ не дает абсолютно никаких гарантий, что получится найти престижную работу. Намного более важное значение имеют практические навыки специалиста, а также его опыт работы. Именно поэтому решение о заказе диплома можно считать целесообразным. Приобрести диплом об образовании mosdmit.flybb.ru/viewtopic.php?f=2&t=636
Pingrutty
| #
darknet market links onion dark website
Toliknaf
| #
darknet markets darknet market
DonDonfaita
| #
darknet markets links darknet links
Donaldlaf
| #
bitcoin dark web darknet markets 2025
Pingrutty
| #
darkmarkets dark markets
Davidhoirm
| #
amazon deals and steals
Toliknaf
| #
bitcoin dark web darknet drug links
prodvijenie saitov v moskve_ejol
| #
продвижение сайта в топ цена москва продвижение сайта в топ цена москва .
prodvijenie saitov v moskve_ytsi
| #
заказать раскрутку сайта заказать раскрутку сайта .
DavidNaw
| #
https://textme.work/
DonDonfaita
| #
darknet markets onion address darknet markets links
Pingrutty
| #
dark market dark market
Donaldlaf
| #
darknet market links onion dark website
prodvijenie saitov v moskve_wzoa
| #
заказать seo заказать seo .
prodvijenie saitov v moskve_ioEl
| #
сео оптимизация москва сео оптимизация москва .
Toliknaf
| #
dark market onion darknet markets 2025
lkjhCype
| #
https://jaluzi-bryansk.ru/smarty/pags/filmu_onlayn_besplatno___dostupnoe_udovolstvie.html metro last light на гитаре табы
Michaelwhild
| #
fps samp гта сан андреас самп 0.3
TylerOwece
| #
сайт крмп самп форум
Pingrutty
| #
dark markets 2025 darknet site
DonDonfaita
| #
best darknet markets darknet market list
Donaldlaf
| #
darknet sites dark web market list
1win_ugSr
| #
1 win http://1win104.com.kg/ .
Toliknaf
| #
darknet markets onion darkmarkets
Pingrutty
| #
darknet marketplace darknet markets 2025
Sazrkbm
| #
Здравствуйте!
Заказать диплом ВУЗа по невысокой стоимости возможно, обращаясь к проверенной специализированной фирме. Заказать диплом о высшем образовании: vacshidiplom.com/kupit-diplom-stavropol-5/
DonDonfaita
| #
darknet site darknet marketplace
Donaldlaf
| #
darkmarket link dark markets
ThomasBem
| #
https://kro-rodina.ru/
mostbet_chPr
| #
мосбет https://mostbet34.com.kg .
Pingrutty
| #
darknet markets links darkmarket link
Toliknaf
| #
darknet markets links bitcoin dark web
1win_josa
| #
1win betting 1win9.com.ng .
DonDonfaita
| #
best darknet markets dark markets
lkjhCype
| #
https://remontila.ru/art/filmu_onlayn_v_luchshem_kachestve.html смотреть фильм волк с уолл стрит бесплатно в хорошем качестве
Donaldlaf
| #
dark markets 2025 darkmarkets
Pingrutty
| #
dark web market list darkmarket list
Terencesam
| #
Бонусы за регистрацию Игровые автоматы играть на деньги
Jasonbitte
| #
Когда я зашёл на этот сайт, ощущение было таким, будто я переступил грань реальности. Здесь каждая ставка — это не просто азарт, а история, которую ты открываешь с каждым кликом.
Оформление создан для комфорта, словно невидимый проводник направляет тебя от игры к игре. Финансовые движения, будь то депозиты или вывод средств, проходят легко, как поток воды, и это завораживает. А поддержка всегда готова подхватить, как надежный товарищ, который никогда не оставит.
Для меня Селектор стал местом, где игра и вдохновение сплетаются. Здесь каждый момент — это часть картины, которую хочется создавать снова и снова.
Toliknaf
| #
dark market url dark web market urls
mostbet_ruPr
| #
мостбет зеркало https://www.mostbet34.com.kg .
DonDonfaita
| #
darknet drug store darknet sites
Davidhoirm
| #
азино777 официальный сайт
Donaldlaf
| #
dark web drug marketplace darknet drug links
Crazimesix
| #
Ищете выгодное предложение? Уникальная возможность получить займы без процентов онлайн на карту от 60+ проверенных МФО. Первый займ бесплатно для новых клиентов! Мы гарантируем полное отсутствие скрытых комиссий и переплат при оформлении микрокредита до 30 000 рублей.
Crazimesix
| #
Впечатляет количество вариантов займа онлайн срочно без отказа среди современных МФО. Срочные 11 000 рублей на медицинские услуги получил быстро благодаря широкому выбору компаний. Удобное оформление и моментальное зачисление денег спасли ситуацию.
Toliknaf
| #
darkmarket link darknet drug links
Pingrutty
| #
darkmarket darknet marketplace
servicecaree
| #
Samsung представила серию ноутбуков Galaxy Book4, в которую входят модели, подходящие для повседневной работы, творчества и бизнес-задач. В данной статье мы разберем две ключевые модели серии — Galaxy Book4 Pro и Galaxy Book4 Pro 360, их особенности, характеристики и отличия. подробнее
DonDonfaita
| #
dark web market list dark websites
Donaldlaf
| #
bitcoin dark web darknet links
Crazimesix
| #
В Яндексе отыскал выгодный вариант взять микрозайм на карту, когда срочно понадобилось 16 000 рублей на покупку техники. Поисковая система предложила множество вариантов – выбрал самое честное предложение, средства поступили моментально.
1win_oxsa
| #
1win betting site 1win betting site .
Toliknaf
| #
dark market onion dark market
Pingrutty
| #
darknet markets onion darknet markets url
DonDonfaita
| #
best darknet markets darknet sites
1win_pzSr
| #
казино 1win https://1win104.com.kg/ .
Donaldlaf
| #
darknet drug market dark web drug marketplace
DannyJef
| #
Najboljse pocitnice apartmani Zabljak! Uzivajte v udobju, svezem zraku in osupljivi pokrajini. Gorske poti, smucanje, izleti in prijetno vzdusje. Brezplacen Wi-Fi, zajtrk in parkirisce. Rezervirajte bivanje v osrcju narave!
Richardbed
| #
Dobrodosli v hotel Bianca Kolasin! Uzivajte v udobnih sobah, osupljivem razgledu in odlicni storitvi. Pozimi – smucanje, poleti – pohodi v gore in izleti. Prirocna lokacija, restavracija, SPA in prijetno vzdusje. Rezervirajte nepozabne pocitnice!
mostbet_sjmt
| #
mostbet 1-15 pozitsiya http://mostbet3003.ru/ .
Pingrutty
| #
dark markets 2025 dark web market list
Cazralz
| #
Здравствуйте!
Мы можем предложить дипломы психологов, юристов, экономистов и любых других профессий по приятным ценам. Стоимость может зависеть от выбранной специальности, года получения и университета: rdiplomans.com/
Uazrpml
| #
Доброго времени суток!
Для определенных людей, купить диплом ВУЗа – это острая необходимость, шанс получить достойную работу. Но для кого-то – это очевидное желание не терять множество времени на учебу в ВУЗе. Что бы ни толкнуло вас на такое решение, мы готовы помочь. Быстро, профессионально и по разумной стоимости изготовим документ любого ВУЗа и года выпуска на подлинных бланках со всеми требуемыми подписями и печатями.
Ключевая причина, почему многие покупают дипломы, – получить хорошую работу. Допустим, знания дают возможность кандидату устроиться на привлекательную работу, однако документального подтверждения квалификации не имеется. Если работодателю важно наличие “корочки”, риск потерять вакантное место достаточно высокий.
Заказать документ о получении высшего образования можно в нашем сервисе. Мы предлагаем документы об окончании любых ВУЗов РФ. Вы сможете получить необходимый диплом по любой специальности, любого года выпуска, включая документы Советского Союза. Даем гарантию, что в случае проверки документов работодателем, никаких подозрений не появится.
Ситуаций, которые вынуждают приобрести диплом ВУЗа очень много. Кому-то срочно необходима работа, в итоге нужно произвести особое впечатление на начальство при собеседовании. Некоторые мечтают устроиться в престижную компанию, чтобы повысить собственный статус в социуме и в последующем начать свое дело. Чтобы не тратить драгоценное время, а сразу начинать успешную карьеру, используя имеющиеся знания, можно купить диплом через интернет. Вы станете полезным в обществе, получите финансовую стабильность в минимальные сроки- купить аттестат
Sazrjhq
| #
Довольно часто бывает так, что для того, чтобы продвигаться вверх по карьере, требуется документ, который подтверждает наличие образования. Где купить диплом специалиста?
Купить документ о получении высшего образования можно у нас. Мы предлагаем документы об окончании любых университетов России. mebcom.ks.ua/
RonaldIcemy
| #
Хотите сдать радиолом https://radiolom99.ru? Мы принимаем микросхемы, платы, процессоры и прочие радиоэлементы по выгодным ценам. Быстрая оценка, честные цены и удобные способы приема. Узнайте, сколько стоят ваши детали прямо сейчас!
Toliknaf
| #
tor drug market dark web market
Sazrwva
| #
Купить диплом о высшем образовании
Мы оказываем услуги по продаже документов об окончании любых университетов РФ. http://www.alternance-manufacturing.fr/contact
DonDonfaita
| #
darknet markets url dark web market list
Sazrmtv
| #
Привет, друзья!
Заказ документа о высшем образовании через проверенную и надежную фирму дарит много достоинств. Данное решение позволяет сэкономить как длительное время, так и существенные финансовые средства. Однако, только на этом выгода не ограничивается, плюсов значительно больше.Мы можем предложить дипломы любой профессии. Дипломы изготавливаются на фирменных бланках. Доступная цена в сравнении с большими расходами на обучение и проживание. Заказ диплома ВУЗа является выгодным шагом.
Приобрести диплом: magic.ly/diplomygroup/Pochemu-stoit-kupit-shkolnyj-attestat-pryamo-sejchas
Donaldlaf
| #
dark web market links darknet market lists
lkjhCype
| #
https://logospress.ru/content/pgs/?filmu_slesheru__istoriya__osobennosti_i_vliyanie_na_kulturu.html новинки кино и сериалы смотреть
Pingrutty
| #
tor drug market darknet markets onion address
Toliknaf
| #
darknet markets links darknet markets onion
DonDonfaita
| #
darknet market dark markets 2025
Pingrutty
| #
dark web drug marketplace dark market url
Donaldlaf
| #
dark web drug marketplace darknet market links
Terencesam
| #
1000 рублей за регистрацию вывод сразу bonuses casinos
lkjhCype
| #
https://panamar.ru/mt/?filmu_onlayn___udobno__dostupno_i_uvlekatelno.html фильм хороший посмотреть для души рейтинг
Toliknaf
| #
darknet market lists darknet markets 2025
Pingrutty
| #
best darknet markets dark market 2025
DonDonfaita
| #
dark websites darknet markets onion
Xazrxip
| #
Приобрести диплом о высшем образовании!
Мы готовы предложить дипломы любых профессий по приятным тарифам. Вы приобретаете диплом через надежную компанию. : diplomers.com/kupit-diplom-gei-o-visshem-obrazovanii-bez-predoplati-3/
Donaldlaf
| #
darknet markets darknet links
servicecaree
| #
Ремонт роботов-пылесосов iRobot в Москве: Ремонт блока питания, Замена комплекта щеток, Восстановление колеса, Ремонт гидросистемы, Калибровка, Замена датчиков, Очистка датчиков, Замена аккумулятора здесь
Toliknaf
| #
dark markets 2025 darknet markets url
Pingrutty
| #
darkmarket list darknet markets 2025
Lazrcgn
| #
Где купить диплом специалиста?
Заказать диплом института по доступной цене можно, обратившись к проверенной специализированной компании.: nsk-diplom.com
WilliamDix
| #
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам. Дипломы изготавливаются на фирменных бланках Купить диплом о высшем образовании diplomp-irkutsk.ru
DonDonfaita
| #
darknet market list darknet market
Donaldlaf
| #
dark market list darkmarket 2025
Pingrutty
| #
onion dark website darknet markets url
Toliknaf
| #
dark market link darkmarket 2025
DonDonfaita
| #
darknet markets url darkmarket url
Donaldlaf
| #
darknet markets tor drug market
Pingrutty
| #
dark web link dark markets 2025
Toliknaf
| #
darknet markets 2025 darkmarkets
Diplomi_ihKt
| #
Заказать диплом о высшем образовании!
Мы готовы предложить документы учебных заведений, расположенных на территории всей Российской Федерации.
Превосходства наших дипломов:
• используем лишь качественные бланки “Гознак”;
• все подписи должностных лиц;
• все печати учебного заведения;
• водяные знаки, нити и прочие степени защиты;
• безупречное качество оформления – ошибок не будет;
• любая проверка оригинальности документа.
bisness-diplom.ru/kupit-diplom-trenera-2-2
DonDonfaita
| #
bitcoin dark web best darknet markets
Pingrutty
| #
darknet market links darknet marketplace
Donaldlaf
| #
darkmarket dark web market
DouglasOrist
| #
Купите теплицу https://tepl1.ru/catalog с доставкой по выгодной цене! Широкий выбор моделей: поликарбонатные, стеклянные, пленочные. Быстрая доставка, прочные конструкции, удобный монтаж. Идеально для дачи, сада и фермерства. Заказывайте качественные теплицы с доставкой прямо сейчас!
Sazrmam
| #
купить диплом невинномысск
Toliknaf
| #
dark web link dark market onion
Pingrutty
| #
dark market url dark web market
DonDonfaita
| #
dark web market links darknet sites
Terencesam
| #
Игровые автоматы играть на деньги фриспины за регистрацию
Donaldlaf
| #
darknet websites darknet links
prodvijenie saitov v moskve_dnOr
| #
заказ продвижения сайта заказ продвижения сайта .
prodvijenie saitov v moskve_pxka
| #
заказать сео продвижение сайта москва заказать сео продвижение сайта москва .
prodvijenie saitov v moskve_wgKr
| #
стоимость продвижения сайта в топ 10 цена prodvizhenie-sajtov-v-moskve214.ru .
prodvijenie saitov v moskve_ejPt
| #
сео заказать сео заказать .
Toliknaf
| #
dark web marketplaces dark web market urls
Pingrutty
| #
darknet markets onion address dark markets
lkjhCype
| #
https://gefestexpo.ru/art/filmu_onlayn___novue_grani_kinoprosmotra.html смотреть фильм и сериалы бесплатно
DonDonfaita
| #
dark web market darknet markets 2025
Donaldlaf
| #
dark market onion darknet markets 2025
1win_agOn
| #
казино 1win https://1win105.com.kg .
Pingrutty
| #
darknet markets url darknet markets
Toliknaf
| #
darknet market lists dark web link
DonDonfaita
| #
darknet markets url dark market list
Donaldlaf
| #
darknet market darknet marketplace
Pingrutty
| #
dark web markets darknet markets
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://xn—-7sbccdscdoffrg1cybj3z.xn--p1ai/
mostbet_dcEr
| #
mostbet apk скачать https://mostbet1009.com.kg/ .
lkjhCype
| #
http://radiodelo.ru/shop/pgs/filmu__professionalu_svoego_dela.html хочу замуж фильм смотреть 2022
Toliknaf
| #
dark market onion dark market onion
1win_jpMl
| #
1 win 1win10.com.ng .
EdwardAbaph
| #
https://inkasbank.ru/
DonDonfaita
| #
darknet drugs darknet sites
Pingrutty
| #
darkmarket url dark markets 2025
Donaldlaf
| #
darknet site darknet drug market
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://xn--90aimcoinaikf.xn--p1ai/
Trefyfa
| #
Приобрести документ университета можно у нас в столице. diplom-ryssia.com/kupit-diplom-orenburg-2
mostbet_jgEr
| #
мостбет авиатор http://mostbet1009.com.kg .
Pingrutty
| #
darkmarket darknet markets links
servicecaree
| #
Подробнее в материале ссылка
DonDonfaita
| #
dark market link dark market url
Donaldlaf
| #
darkmarket dark market onion
Sazrzrp
| #
Приобретение документа о высшем образовании через качественную и надежную компанию дарит ряд плюсов. Такое решение помогает сэкономить как дорогое время, так и значительные деньги. Однако, плюсов значительно больше.Мы готовы предложить дипломы любой профессии. Дипломы производят на фирменных бланках. Доступная стоимость по сравнению с огромными тратами на обучение и проживание. Заказ диплома о высшем образовании из российского института является мудрым шагом.
Заказать диплом: diplom-zentr.com/kupit-diplom-v-moskve-bistro-i-nadezhno-3/
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://xn——6cdbaabncc2b3blicg2a2b7axf0dzfui.xn--p1ai/
https://Menbehealth.Wordpress.com/
| #
It’s remarkable to visit this site and reading the views
off all friends concerning this piece of writing, while
I am also zealous of getting knowledge. https://Menbehealth.Wordpress.com/
Terencesam
| #
бонусы за регистрацию Играть игровые автоматы на деньги
Pingrutty
| #
onion dark website dark market list
MarkNOlaf
| #
bitcoin dark web https://drdarkfoxmarket.com – dark market onion
DonDonfaita
| #
dark market 2025 dark web market list
FNDaviditabe
| #
darknet market https://kingdom-marketplace.com/ – darkmarket url
WilliamIRrutty
| #
darkmarkets https://kingdomdarkmarketonline.com/ – darknet site
Donaldlaf
| #
darknet markets url dark markets
Markcen
| #
Хотите сдать квартиру в аренду? Читайте проверенные советы https://xn—-8sbqbliyb2m.xn--p1ai/
Kxyufaita
| #
darknet drug store https://cannahome-darkmarket-online.com/ – dark web market list
Pingrutty
| #
darknet drugs darknet drug store
1win_mvOn
| #
1 вин вход http://1win105.com.kg/ .
lkjhCype
| #
https://www.evacuator-moskva.ru/images/pages/index.php?gangsterskoe_kino__istoriya__shedevru_i_kulturnoe_vliyanie.html фильмы онлайн скачать на телефон
servicecaree
| #
Подробнее в материале перейти
DonDonfaita
| #
darknet drug links darknet market
1win_dlMl
| #
sports betting 1win sports betting 1win .
Donaldlaf
| #
dark web market urls darknet drug store
Pingrutty
| #
darkmarket list darknet market lists
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://xn—–7kchcrgop2a1bbbeqft7m5b.xn--p1ai/
Jasonbitte
| #
Когда я зашёл на этот сайт, чувство было таким, будто я переступил грань реальности. Здесь каждая ставка — это не просто шанс, а эмоция, которую ты проживаешь с каждым движением.
Дизайн создан для комфорта, словно ветер судьбы направляет тебя от игры к игре. Транзакции, будь то пополнения или вывод средств, проходят быстро, как поток воды, и это удивляет. А техподдержка всегда отвечает мгновенно, как верный помощник, который никогда не подведёт.
Для меня Селектор стал пространством, где игра и вдохновение переплетаются. Здесь каждая минута — это часть картины, которую хочется создавать снова и снова.
DonDonfaita
| #
dark web drug marketplace darknet drugs
Pingrutty
| #
dark market onion darkmarket
Donaldlaf
| #
darknet drug links darknet websites
oformit propysk v Moskvy_djEi
| #
оформить пропуск в Москву progorod43.ru/oformit-propusk-na-mkad-dlya-gruzovyh-mashin .
servicecaree
| #
Подробнее в материале на сайте
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://xn—–7kcoqeea0aaiqm4apq0s.xn--p1ai/
Jariorfij
| #
Привет!
Руководители серьезных компаний очень часто выбирают претендентов, которые закончили университет. Особенно ценятся топовые заведения. Но учиться 5 лет – это долго и дорого, далеко не у каждого существует подобная возможность. Купить документ – лучший выход.
Бывают и непредвиденные обстоятельства, когда диплом теряется или портится. Не всегда возможно быстро и без проблем восстановить документ, особенно когда университет закрыт или располагается в другом регионе страны. Бюрократия отнимает много времени.
Для максимально быстрого продвижения по карьерной лестнице нужно наличие официального диплома о высшем образовании. Впрочем зачастую в жизни может случиться так, что определенные трудности не дают с успехом окончить учебу, получая желанный документ.
Купить диплом о высшем образовании
Наши специалисты предлагают выгодно приобрести диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, водяными знаками, подписями. Документ способен пройти любые проверки, даже при помощи профессионального оборудования. Достигайте своих целей быстро и просто с нашим сервисом.
Где купить диплом по необходимой специальности? rdiploma24.com/
1win_ansn
| #
казино 1win http://www.1win106.com.kg .
Pingrutty
| #
darknet markets 2025 dark web link
DonDonfaita
| #
darknet markets onion address dark market list
Donaldlaf
| #
dark web market dark market 2025
HershelzeM
| #
פנים! ויקה, אגב, גם ביקשה להסיר אותה. אתה לא מפחד ממה שהיא תראה? – יש מקומות כאלה בטלפון שבו היא בהחלט לא תמצא. בחיוך ענה מקס. – אותם בעוד שבוע? – מה שתגיד, יקירתי. אני יכול להזמין גם שלושה. – נהדר! שיהיה משהו חדש. – כן, מותק. גם אני חושב כך. כן, מרטין, browse this site
RolandLob
| #
https://poufe.ru/topic.php?id=1567
VortexHunter
| #
Современные методы по бизнесу: https://student.uog.edu.et/hello-world-3/
Markcen
| #
Хотите сдать квартиру в аренду? Читайте проверенные советы https://xn—-7sbhyegsibavlngp.xn--p1ai/
Pingrutty
| #
dark websites darkmarket
Eanrqeo
| #
Для успешного продвижения по карьере необходимо наличие диплома о высшем образовании. Купить диплом любого института у сильной организации: diplomidlarf.ru/kupit-diplom-parikmaxera-12/
Terencesam
| #
bonuses casinos фриспины за регистрацию
Edwardmeafe
| #
зеркало armada casino
RolandLob
| #
https://cross.expert/partner/mrt-pozvonochnika-vazhnyy-metod-diagnostiki.html
DonDonfaita
| #
bitcoin dark web onion dark website
Edwardmeafe
| #
армада
servicecaree
| #
Подробнее в материале ссылка
Donaldlaf
| #
dark web sites darknet markets onion address
mostbet_kg_xdpi
| #
служба поддержки мостбет номер телефона http://mostbet1000.com.kg .
Pingrutty
| #
darkmarket 2025 darknet site
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://xn--j1aihe7cc.com/
MarkNOlaf
| #
darkmarket link https://dark-web-versus.com – darknet markets onion
DonDonfaita
| #
dark market onion darknet drugs
mostbet_besa
| #
скачать mostbet на телефон https://www.mostbet1010.com.kg .
Donaldlaf
| #
darknet links dark web market
Pingrutty
| #
darknet drug links darknet markets onion address
FNDaviditabe
| #
darknet markets url https://kingdom-marketplace.com/ – dark web market urls
WilliamIRrutty
| #
darknet markets https://asap-market-onion.com/ – onion dark website
Potolki_dex
| #
натяжной потолок цена https://potolki-v-moskve.ru/
Kxyufaita
| #
darkmarket list https://cyberdarkmarkets.com/ – darknet markets onion
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://an-rusdom.ru/
Potolki_dex
| #
натяжной потолок москва https://potolki-v-moskve.ru/
MichaelBluth
| #
Sans?n?z? Sweet Bonanza’da sweet-bonanza-casino.site deneyin – parlak ve karl? bir slot! Ucretsiz donusler, kazanc carpanlar?, kademeli kombinasyonlar ve buyuk odemeler. Ucretsiz veya parayla cevrimici oynay?n ve tatl? oduller kazan?n!
Martinownew
| #
Glory Casino’da glory-casino-guncel-giris.online oynayn – en iyi oyunlar, yuksek odemeler ve 7/24 destek! Populer slotlar?, kart oyunlar?n? ve ruleti secin, bonuslar kazan ve gercek para kazan?n!
VirgilNeali
| #
Diego Armando Maradona diego maradona es una leyenda del futbol mundial! Campeon del Mundo de 1986, autor de “La Mano de Dios” y “El Gol del Siglo”. Un gran jugador argentino que inspiro a generaciones. ?Su tecnica, su regate y su pasion por el juego lo convirtieron en un icono del futbol!
mobile-worker.ru
| #
Ваш надёжный помощник снова в строю! Профессиональная замена аккумулятора iphone 8 вернёт смартфону автономность. Оригинальные комплектующие и современное оборудование – залог качественного обслуживания вашего устройства.
Более 7 лет наш сервисный центр на Коломенской помогает москвичам решать технические проблемы. В августе 2022 года Mobile-Worker переехал в новое просторное помещение на проспекте Андропова, 17к1. Теперь наши клиенты могут наслаждаться ещё более комфортным обслуживанием.
mostbet_kg_napi
| #
мостбет зеркало мостбет зеркало .
Онлайн казино в Беларуси
| #
Легальные Онлайн казино в Беларуси Беларуси
предлагают игровые автоматы от
мировых производителей
DonDonfaita
| #
darknet websites bitcoin dark web
MarkNOlaf
| #
darknet market links https://dark-web-versus.com – dark markets
Pingrutty
| #
darknet links darknet marketplace
Donaldlaf
| #
darkmarket url darknet markets onion address
FNDaviditabe
| #
darkmarket https://kingdom-marketplace.com/ – darknet drugs
WilliamIRrutty
| #
dark market url https://asap-market-onion.com/ – dark web drug marketplace
Kxyufaita
| #
dark web market links https://darkfoxmarketplace.com/ – dark web markets
DarkVoyager
| #
Лучшие https://aacsatlanta.com/dui-school-marietta/ инструменты для работы с бизнесом.
mobile-worker.ru
| #
Наконец-то нашёл отличный сервис для ремонт геймпада! После года интенсивного использования джойстики стали заедать. Мастера быстро определили проблему и заменили изношенные детали. Теперь играю с удовольствием, как на новом контроллере!
Mobile-Worker – это команда профессионалов с опытом от 7 лет, которые относятся к каждому устройству как к своему собственному. Мы работаем со всеми популярными брендами: iPhone, Samsung, Xiaomi и другими. Наш адрес: проспект Андропова, 17к1, работаем ежедневно с 10:00 до 20:00.
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://bti-nsk.ru/
Pingrutty
| #
darknet market darknet links
DonDonfaita
| #
darknet links bitcoin dark web
MarkNOlaf
| #
dark web marketplaces https://drdarkfoxmarket.com – darknet drug market
Donaldlaf
| #
dark markets darknet drug store
FNDaviditabe
| #
darkmarket 2025 https://firstdarkmarket.com/ – darknet market links
Cazrvct
| #
Мы готовы предложить дипломы любых профессий по доступным ценам. Купить аттестат 9 классов — kyc-diplom.com/attestat-9-klassov.html
WilliamIRrutty
| #
dark web link https://kingdommarketdarknet.com/ – darknet site
Cazrxmu
| #
Мы готовы предложить документы ВУЗов на Ваш выбор, которые расположены в любом регионе России. nsk-diplom.com/kupit-diplom-baltijskoj-akademii-turizma-i-predprinimatelstva-3
1win_nosn
| #
1вин вход http://1win106.com.kg/ .
Kxyufaita
| #
darkmarket url https://cannahome-darkmarket-online.com/ – darknet market list
Pingrutty
| #
dark web drug marketplace dark web link
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://crystalit34.ru/
ManuelGlona
| #
Fansite de Mike Tyson https://mike-tyson.com.mx donde podras conocer su historia, peleas, noticias y todo lo relacionado a su legado boxistico. Sigue sus proyectos y conoce mas sobre esta leyenda.
JamesGom
| #
Sitio de fans de Michael Phelps michael-phelps.com.mx Un lugar donde los fanaticos pueden encontrar toda la informacion sobre su carrera, sus records y su vida. Noticias, logros y contenido exclusivo.
DenniscrypE
| #
Sitio de fans de Muhammad Ali https://muhammad-ali.com.mx La historia de una leyenda del boxeo, sus victorias, su lucha por sus derechos y su legado que inspiro al mundo.
DonDonfaita
| #
dark web drug marketplace darkmarket 2025
MarkNOlaf
| #
dark websites https://kingdomdarkmarketplace.com – dark web marketplaces
mostbet_bgsa
| #
mostber https://mostbet1010.com.kg .
Donaldlaf
| #
darknet drug store dark web market
FNDaviditabe
| #
dark web market https://firstdarkmarket.com/ – darknet markets 2025
WilliamIRrutty
| #
onion dark website https://asap-market-onion.com/ – darknet markets url
Terencesam
| #
бонусы за регистрацию Casino bonus
Pingrutty
| #
darkmarket url dark market list
Kxyufaita
| #
dark markets https://cyberdarkmarkets.com/ – darknet drug links
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://cspkaliningrad.ru/
MarkNOlaf
| #
darknet drug market https://kingdom-darkmarketplace.com – dark market link
DonDonfaita
| #
darknet links darknet links
ClaudeKen
| #
Sitio fan de George Foreman http://george-foreman.com.mx dedicado a su carrera, victorias, derrotas y legado en el boxeo. Historias inspiradoras y el estilo unico de una leyenda del deporte.
Donaldlaf
| #
dark market url darknet drug links
Pingrutty
| #
dark market link darknet market links
FNDaviditabe
| #
dark web drug marketplace https://onion-dark-market.shop/ – dark web market links
WilliamIRrutty
| #
dark market list https://asap-market-onion.com/ – dark web market list
IronX1
| #
Как сэкономить на бизнесе: https://karoen.nl/blog/2023/06/06/ontspan-en-geniet-een-gids-voor-de-perfecte-vakantie/
Kxyufaita
| #
darknet drug links https://cannahomemarket.link/ – dark web market
Markcen
| #
Хотите сдать квартиру в аренду? Читайте проверенные советы https://dachnoe-house.ru/
MarkNOlaf
| #
dark web markets https://cannahomedarknetdrugstore.com/ – dark market list
Pingrutty
| #
darknet markets onion address darknet site
Donaldlaf
| #
dark market link darknet drug store
Volodyanaf
| #
dark web market https://aasap-market.com/ – darknet site
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://fisher-kp.ru/
FNDaviditabe
| #
darkmarket 2025 https://firstdarkmarket.com/ – darkmarket list
WilliamIRrutty
| #
dark web market https://kingdommarketdarknet.com/ – darknet drug links
Pingrutty
| #
darknet markets tor drug market
DonDonfaita
| #
dark markets darkmarket link
Kxyufaita
| #
darknet markets onion address https://cannahome-darkmarket-online.com/ – dark markets 2025
MatthewsOf
| #
Всегда жду выхода новых сезонов любимых зарубежных сериалов Последний С…РѕРґ королевы (сериал 2015, РўР’Р¦) смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
Yi Chapmond
| #
Na [580bet](https://580-bet.com), o primeiro passo para uma experiência de cassino incrível começa com um bônus de US$ 100! Ao se registrar como novo usuário, você receberá esse valor para apostar em jogos emocionantes. Aproveite a oportunidade de explorar tudo o que a plataforma tem a oferecer, com segurança e diversão.
Donaldlaf
| #
darknet site bitcoin dark web
MarkNOlaf
| #
darkmarket https://kingdomdarkmarketplace.com – darknet drug market
MatthewsOf
| #
Лучшие русские сериалы – это настоящая гордость кинематографа Лучшие СЂСѓСЃСЃРєРёРµ сериалы смотреть онлайн бесплатно РІ хорошем качестве » Страница 721
Volodyanaf
| #
dark web market list https://darkfoxdarkweb.com/ – darkmarket
MatthewsOf
| #
Зарубежные сериалы иногда просто поражают крутыми сюжетными поворотами Чужое счастье (сериал 2017, Р РѕСЃСЃРёСЏ) смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
MatthewsOf
| #
Какие русские сериалы сейчас на пике популярности? Стюардесса (фильм 2018) смотреть онлайн бесплатно РІ хорошем качестве
LightZ4
| #
https://nettyfish.com/bulk-sms-service-in-chennai/ Подробное руководство по бизнесу.
Pingrutty
| #
dark web marketplaces darkmarkets
FNDaviditabe
| #
dark web market https://firstdarkmarket.com/ – best darknet markets
WilliamIRrutty
| #
darknet drug store https://idarknetmarket.com/ – dark web drug marketplace
DonDonfaita
| #
darkmarket link dark market url
MatthewsOf
| #
Русские комедийные сериалы стали гораздо лучше за последние годы Честное волшебное (фильм 1975) смотреть онлайн бесплатно РІ хорошем качестве
Dolly Herzfeld
| #
Com o app [aposte e ganhe](https://aposte-br.com), você pode apostar onde quiser. Instale o aplicativo facilmente e aproveite a interface otimizada, gráficos de alta qualidade e uma experiência de jogo excelente diretamente no seu celular.
MatthewsOf
| #
Зарубежные комедийные сериалы – отличный способ поднять настроение Ёлки 3 (фильм 2013) смотреть онлайн бесплатно РІ хорошем качестве
Kxyufaita
| #
dark markets https://cannahome-darkmarket-online.com/ – dark web market
Donaldlaf
| #
dark web markets darkmarket url
MatthewsOf
| #
Зарубежные сериалы по книгам часто бывают очень интересными РЇ тебя услышу 1 сезон 18 серия смотреть онлайн бесплатно РІ хорошем качестве РЅР° СЂСѓСЃСЃРєРѕРј языке
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://hertz-lumen.ru/
MatthewsOf
| #
Русские сериалы о молодежи стали очень качественными Мюзиклы смотреть онлайн бесплатно РІ хорошем качестве HD без регистрации » Страница 2
MarkNOlaf
| #
darknet markets onion https://dark-web-versus.com – dark web market urls
Volodyanaf
| #
darkmarket 2025 https://darkfoxdarkweb.com/ – darknet market links
Pingrutty
| #
dark market 2025 dark web market urls
Terencesam
| #
получить 1000 рублей бесплатно за регистрацию no deposit casino
Sazrizy
| #
Хочу поделиться своим опытом по заказу аттестата ПТУ. Думал, что это невозможно, и начал искать информацию в интернете по теме: купить диплом риэлтора, купить диплом слесаря ремонтника дпо стандарт, купить диплом слесаря сантехника, купить диплом строительного техникума ссср, купить диплом сыктывкар. Постепенно углубляясь в тему, нашел отличный ресурс здесь: diplomybox.com/zakazat-dokument
obzorcaree
| #
Подробнее в материале в блоге об обзорах электронной техники перейти
MatthewsOf
| #
Какой зарубежный сериал стоит посмотреть на выходных? Сильная личность РёР· 2-Рђ (фильм 1984) смотреть онлайн бесплатно РІ хорошем качестве
FNDaviditabe
| #
darknet market list https://kingdom-marketplace.com/ – dark market list
MatthewsOf
| #
Лучшие русские сериалы – это те, которые цепляют с первых минут Драмы смотреть онлайн бесплатно РІ хорошем качестве HD без регистрации » Страница 47
WilliamIRrutty
| #
darknet sites https://darknetmarketsonion.shop/ – dark web link
MatthewsOf
| #
Какие русские сериалы можно назвать культовыми? Темнее чёрного (аниме 2007-2010) РЅР° СЂСѓСЃСЃРєРѕРј языке смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
DonDonfaita
| #
best darknet markets dark market 2025
MatthewsOf
| #
Какие русские сериалы сейчас на пике популярности? Гром ярости (фильм 2010, РќРўР’) смотреть онлайн бесплатно РІ хорошем качестве
Kxyufaita
| #
best darknet markets https://cyberdarkmarkets.com/ – darknet site
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные рекомендации — https://homedecorsochi.ru/
MatthewsOf
| #
Лучшие русские сериалы – это те, которые вызывают бурю эмоций Мультсериалы смотреть онлайн бесплатно, РЅРѕРІРёРЅРєРё мультсериалов РІ HD качестве » Страница 22
Donaldlaf
| #
darknet markets url dark web marketplaces
Jacobisord
| #
стоимость проката авто прокат машин сочи без водителя
Pingrutty
| #
darkmarket url darkmarkets
MichaelGah
| #
Играйте в Big Bamboo big-bamboo-slot.online слот с захватывающим геймплеем, фриспинами и множителями! Ощутите атмосферу восточной удачи, собирайте бонусные символы и выигрывайте по-крупному.
oformit propysk v Moskvy_oooi
| #
заказать продвижение сайта заказать продвижение сайта .
BruceTam
| #
Join Glory Casino glory-casino-bd.online and get maximum bonuses! Top slot machines, table games, jackpots and exclusive promotions. Play online and win comfortably!
oformit propysk v Moskvy_hxet
| #
seo продвижение сайта москва seo продвижение сайта москва .
BufordClada
| #
Гемблинг-клуб Вулкан – это захватывающий мир азарта, который впечатляет с первого взгляда. Благодаря шикарному дизайну и интуитивно понятному интерфейсу, пользователи быстро оказываются в этом игровом пространстве.
Среди сильных сторон казино игровые автоматы стоит отметить большой выбор игр, включая игровые автоматы и настольные азартные игры, которые подойдут каждому игроку. Качество графического дизайна и анимации делает игровой процесс больше увлекательным и захватывающим.
Однако больше всего поразил подход казино к безопасности игроков. Они используют новейшие технологии шифрования, чтобы обеспечить секретность личных и финансовых данных пользователей. Это делает казино Вулкан замечательным вариантом для онлайн-игры.
MarkNOlaf
| #
dark web market links https://kingdomdarkmarketplace.com – darknet markets
Volodyanaf
| #
dark web sites https://darkfoxdarkweb.com/ – darknet drug market
Dnrtqnt
| #
Где заказать диплом по необходимой специальности?
Мы изготавливаем дипломы любой профессии по выгодным тарифам. Мы можем предложить документы техникумов, расположенных в любом регионе России. Вы можете купить качественно напечатанный диплом за любой год, включая сюда документы старого образца СССР. Дипломы и аттестаты выпускаются на “правильной” бумаге самого высокого качества. Это позволяет делать настоящие дипломы, которые невозможно отличить от оригиналов. Они будут заверены всеми обязательными печатями и штампами. Стараемся поддерживать для клиентов адекватную ценовую политику. Для нас очень важно, чтобы дипломы были доступны для большого количества наших граждан. diplom5.com/kupit-diplom-shvei
MatthewsOf
| #
Какие зарубежные сериалы можно назвать лучшими в 2024 году? Сказание РѕР± обручальных кольцах (аниме 2024) РЅР° СЂСѓСЃСЃРєРѕРј языке смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
GhostZ4
| #
Новые подходы в бизнесе: http://traverseearth.com/the-chianti-region-tuscany-italy/
Josephslate
| #
Купить водительские права
FNDaviditabe
| #
dark web marketplaces https://mydarkmarketlink.com/ – darknet markets links
WilliamIRrutty
| #
darkmarkets https://idarknetmarket.com/ – dark markets 2025
MatthewsOf
| #
Зарубежные сериалы – это возможность окунуться в другой мир HD исторические фильмы смотреть онлайн РІ хорошем качестве бесплатно » Страница 31
Pingrutty
| #
dark web market urls darknet market list
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://instrument75.ru/
MatthewsOf
| #
Лучшие зарубежные сериалы – это настоящие блокбастеры Рстория девятихвостого лиса 1938 (дорама 2023) РІСЃРµ серии РїРѕРґСЂСЏРґ смотреть онлайн бесплатно РІ хорошем качестве РЅР° СЂСѓСЃСЃРєРѕРј языке
Kxyufaita
| #
darkmarket list https://cyberdarkmarkets.com/ – dark web market links
Donaldlaf
| #
dark market url darknet links
Calvinweria
| #
https://www.llanelliherald.com/
MatthewsOf
| #
Лучшие зарубежные сериалы – это те, которые становятся легендами Стряпуха. Таланты Рё поклонники (сериал 2024, РўР’Р¦) смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
Volodyanaf
| #
darknet markets 2025 https://aasap-market.com/ – darknet sites
MarkNOlaf
| #
darknet drugs https://kingdomdarkmarketplace.com – darknet sites
Louisa Garding
| #
[74 bet](https://74-bet-br.com): Descubra como sacar seus ganhos rapidamente, com todas as informações necessárias e sem preocupações.
MatthewsOf
| #
Где можно смотреть зарубежные сериалы с хорошим переводом? Невинный (турецкий сериал 2017) РЅР° СЂСѓСЃСЃРєРѕРј языке смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
1win_zaSi
| #
1vin kg 1win107.com.kg .
Pingrutty
| #
tor drug market dark web sites
DonDonfaita
| #
darkmarket url dark web sites
Juliusdaync
| #
Взрослый вебкам бесплатно https://hotcams.one Тысячи моделей, приватные шоу, видео в высоком качестве и горячие трансляции. Общайтесь в чате и наслаждайтесь без ограничений!
FNDaviditabe
| #
darkmarket list https://firstdarkmarket.com/ – dark market 2025
MatthewsOf
| #
Зарубежные комедийные сериалы – отличный способ поднять настроение Смотреть Сериалы 2015 онлайн бесплатно РІ HD качестве » Страница 5
WilliamIRrutty
| #
darkmarket link https://kingdomdarkmarketonline.com/ – darknet drug links
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://kvartiranahodynke.ru/
Donaldlaf
| #
darkmarkets dark web marketplaces
Kxyufaita
| #
dark markets https://cannahome-darkmarket-online.com/ – darkmarkets
lkjhCype
| #
http://stoljar.ru/wp-content/pages/luchshie_filmu_33.html скачать хороший фильм на телефон бесплатно
Pingrutty
| #
darknet websites dark market
Volodyanaf
| #
dark web drug marketplace https://darkwebversus.com/ – darkmarket url
MarkNOlaf
| #
darkmarket link https://kingdomdarkmarketplace.com – darknet market list
DonDonfaita
| #
darknet drugs dark web markets
MatthewsOf
| #
Зарубежные комедийные сериалы – отличный способ поднять настроение Смотреть онлайн бесплатно детективные сериалы, фильмы РІ хорошем качестве » Страница 10
FNDaviditabe
| #
darknet drugs https://onion-dark-market.shop/ – darknet markets
Donaldlaf
| #
dark market onion darkmarket list
WilliamIRrutty
| #
tor drug market https://asap-market-onion.com/ – tor drug market
mostbet_nvSl
| #
mostbet chrono https://mostbet1001.com.kg .
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://lume42.ru/
Larry Hulstine
| #
No site da [winbrl](https://winbrl-88.com), o registro é fácil e rápido, e você recebe um bônus de US$ 100 para começar sua jornada no cassino online. Após completar o cadastro, basta fazer login e aproveitar os melhores jogos de cassino, como slots, roleta e blackjack, com a vantagem do bônus.
Hunter9905
| #
Новые подходы в бизнесе: https://www.editions-ric.fr/2019/02/10/video-france-3-reportage-sur-patrick-lecointe-editions-ric/
Pingrutty
| #
darkmarkets darknet drug market
obzorcaree
| #
Подробнее в материале в блоге об обзорах электронной техники ссылка
Kxyufaita
| #
dark market 2025 https://cyberdarkmarkets.com/ – darknet markets onion
MatthewsOf
| #
Русские комедийные сериалы стали гораздо лучше за последние годы Маша Рё Шеф (шоу 2020) смотреть онлайн РІСЃРµ выпуски РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
oformit propysk v Moskvy_xgOt
| #
сео оптимизация сайтов в москве сео оптимизация сайтов в москве .
MatthewsOf
| #
Зарубежные сериалы в хорошем качестве – это просто сказка Побочный эффект (фильм 2008) смотреть онлайн бесплатно РІ хорошем качестве
MatthewsOf
| #
Какие зарубежные сериалы можно назвать культовыми? Вера (сериал 2024, РўР’-3) смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
Volodyanaf
| #
dark market link https://darkode-market.com/ – dark web link
MatthewsOf
| #
Где смотреть русские сериалы без рекламы и в хорошем качестве? Дом для РґРІРѕРёС… (фильм 2009) смотреть онлайн бесплатно РІ хорошем качестве
MarkNOlaf
| #
darknet links https://drdarkfoxmarket.com – darkmarket 2025
MatthewsOf
| #
Люблю пересматривать старые русские сериалы – в них есть особая атмосфера Красный змей (фильм 2003) смотреть онлайн бесплатно РІ хорошем качестве РЅР° СЂСѓСЃСЃРєРѕРј языке
1win_trEi
| #
cod promoțional 1win http://www.1win5000.ru .
DonDonfaita
| #
dark market 2025 darkmarkets
MatthewsOf
| #
Зарубежные сериалы с крутым сюжетом – это то, что я люблю Фантастические сериалы смотреть онлайн бесплатно РІ хорошем качестве HD без регистрации » Страница 15
lkjhCype
| #
https://adyghe.ru/manul/inc/?filmu_onlayn_na_kinogo_3.html pci ven 8086 dev 43e0
FNDaviditabe
| #
darkmarkets https://firstdarkmarket.com/ – dark web markets
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://mastera-taganroga.ru/
WilliamIRrutty
| #
dark market link https://darknetmarketsonion.shop/ – darknet market links
KennethDrula
| #
Купить права Москва
MatthewsOf
| #
Русские сериалы о молодежи стали очень качественными Поющий офис (шоу 2023) РІСЃРµ выпуски РїРѕРґСЂСЏРґ смотреть онлайн бесплатно РІ хорошем качестве
Katerine Jaramillo
| #
A [bet 7k](https://bet-7k.com) facilita seu acesso ao mundo das apostas online com um app fácil de baixar e instalar. A interface do aplicativo foi projetada para proporcionar uma experiência rápida e eficiente, garantindo que você aproveite ao máximo cada aposta feita na plataforma.
Lezaimus
| #
займ мгновенно на карту – нужен срочный способ получить деньги? Этот сервис предлагает моментальные займы на карту. Без залога и поручителей, решение принимается в течение часа.
Kxyufaita
| #
darkmarket 2025 https://kingdomdarknetdrugstore.com/ – dark web sites
MatthewsOf
| #
Где можно смотреть зарубежные сериалы с хорошим переводом? Путь Рє сердцу мужчины смотреть онлайн бесплатно РІ хорошем качестве РЅР° СЂСѓСЃСЃРєРѕРј языке
Davidhip
| #
крым отдых в крыму
MatthewsOf
| #
Русские сериалы приятно удивляют качеством и интересными сюжетами Тайны дворцовых переворотов (сериал 2000) смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
MatthewsOf
| #
Лучшие русские сериалы – это настоящая гордость кинематографа Смотреть онлайн бесплатно детективные сериалы, фильмы РІ хорошем качестве » Страница 118
Volodyanaf
| #
darknet markets onion address https://darkwebversus.com/ – darknet market
MarkNOlaf
| #
dark web link https://kingdom-darkmarketplace.com – darkmarket list
MatthewsOf
| #
Русские исторические сериалы заслуживают внимания Падение Берлина (сериал 1949) смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
mostbet_esSl
| #
мостбет скачать бесплатно мостбет скачать бесплатно .
obzorcaree
| #
Подробнее в материале в блоге об обзорах электронной техники перейти
Terencesam
| #
bonuses casinos 1000 рублей за регистрацию вывод сразу без вложений
FNDaviditabe
| #
dark web link https://darkmarketdarkfox.com/ – dark web market links
Markcen
| #
Покупка квартиры без рисков? Полезные статьи и советы — https://moderntechnology22.ru/
WilliamIRrutty
| #
darknet drug market https://idarknetmarket.com/ – dark web sites
Terry Berdes
| #
[bingo](https://bingo-br.com): Atendimento ao cliente que resolve suas dúvidas com rapidez, a qualquer momento.
MatthewsOf
| #
Лучшие зарубежные сериалы – это те, которые становятся легендами РўРќРў сериалы смотреть онлайн РЅРѕРІРёРЅРєРё РІСЃРµ серии РїРѕРґСЂСЏРґ РІ хорошем качестве HD без регистрации
Lezaimus
| #
взять кредит на карту без отказа срочно – не бойтесь отказов! Этот сервис работает с любыми клиентами. Простая регистрация, моментальное одобрение. Деньги на карту без проверок.
SteelSlayer
| #
Какие изменения в бизнесе? https://www.tandem.edu.co/grados-2022-1/
Kxyufaita
| #
darknet markets links https://cannahomemarket.link/ – dark markets 2025
Volodyanaf
| #
dark markets https://darkmarketpremium.com/ – darknet market
Zoe Franeo
| #
Bônus de US$ 100 ao se cadastrar na [doce](https://doce-br.com) – cadastre-se já!
MarkNOlaf
| #
darkmarket https://kingdom-darkmarketplace.com – dark market 2025
JamesMeerb
| #
набрать безопасно
MatthewsOf
| #
Всегда жду выхода новых сезонов любимых зарубежных сериалов Мелодрамы смотреть онлайн бесплатно РІ хорошем качестве HD » Страница 276
MatthewsOf
| #
Зарубежные сериалы бывают настолько захватывающими, что невозможно оторваться Сериалы онлайн смотреть бесплатно, РЅРѕРІРёРЅРєРё сериалов РІ HD » Страница 37
MatthewsOf
| #
Какие зарубежные сериалы можно назвать лучшими в 2024 году? Западня (сериал 2024, РўР’Р¦) смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
JamesMeerb
| #
набрать дешево
MatthewsOf
| #
Лучшие русские сериалы – это те, которые заставляют задуматься Сериалы онлайн смотреть бесплатно, РЅРѕРІРёРЅРєРё сериалов РІ HD » Страница 25
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://monolitrealestate.ru/
FNDaviditabe
| #
darknet markets url https://kingdom-marketplace.com/ – darknet markets onion address
WilliamIRrutty
| #
bitcoin dark web https://asap-market-onion.com/ – darkmarket list
Sazrchx
| #
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по выгодным тарифам. Стоимость зависит от выбранной специальности, года выпуска и образовательного учреждения. Всегда стараемся поддерживать для покупателей адекватную ценовую политику. Для нас очень важно, чтобы дипломы были доступными для большого количества наших граждан. купить диплом москва высокого
MatthewsOf
| #
Какой зарубежный сериал стоит начать смотреть прямо сейчас? Версия (сериал 2015, Р РѕСЃСЃРёСЏ) смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве РЅР° СЂСѓСЃСЃРєРѕРј языке
1win_biEi
| #
1win md 1win md .
1win_ztSi
| #
1вин вход http://1win107.com.kg/ .
Kxyufaita
| #
darkmarket list https://cannahomemarket.link/ – darknet market links
MatthewsOf
| #
Люблю сериалы, в которых много неожиданных поворотов Марьина роща (сериал 2014, Р РѕСЃСЃРёСЏ) 1, 2 сезон смотреть онлайн РІСЃРµ серии РїРѕРґСЂСЏРґ бесплатно РІ хорошем качестве
Volodyanaf
| #
dark market list https://aasap-market.com/ – best darknet markets
CharlesSpolf
| #
Witamy w Slottica! Nasza platforma to harmonijne połączenie innowacji, różnorodności i najwyższych standardów bezpieczeństwa, zaprojektowane z myślą o wymagających graczach z Polski. Oferujemy nie tylko szeroki wybór gier – od dynamicznych automatów, przez strategiczne gry stołowe, po ekskluzywne sesje z live dealerami – ale także kompleksowe rozwiązania dostosowane do Twoich preferencji. Dzięki zaawansowanej technologii mobilnej, ciesz się płynnym dostępem do ulubionych rozgrywek na dowolnym urządzeniu, gdziekolwiek jesteś. Slottica Casino współpracuje wyłącznie z uznanymi dostawcami oprogramowania, gwarantując najwyższą jakość grafiki, uczciwość mechaniki i regularne aktualizacje biblioteki gier.
MarkNOlaf
| #
darknet marketplace https://drdarkfoxmarket.com – best darknet markets
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные статьи и советы — https://new-fstore.ru/
MatthewsOf
| #
Лучшие зарубежные сериалы – это те, которые заставляют думать Шахта фильм смотреть онлайн бесплатно РІ хорошем качестве РЅР° СЂСѓСЃСЃРєРѕРј языке
FNDaviditabe
| #
dark web drug marketplace https://firstdarkmarket.com/ – darkmarket url
WilliamIRrutty
| #
onion dark website https://kingdomdarkmarketonline.com/ – darknet links
Travisbub
| #
іменини соломії церковний календар
Janean Steely
| #
Como se registrar na [bet 7k](https://bet-7k.com) e receber seu bônus de US$ 100!
Kxyufaita
| #
darknet sites https://cannahomemarket.link/ – darkmarkets
Storm_Master
| #
Какой https://www.tandem.edu.co/halloween/ вариант предпочесть в бизнесе?
MatthewsOf
| #
Какой зарубежный сериал стоит посмотреть на выходных? РћРґРёРЅ настоящий день (фильм 2022) смотреть онлайн бесплатно РІ хорошем качестве
Volodyanaf
| #
dark web market links https://darkode-market.com/ – darknet market
MatthewsOf
| #
Где можно найти подборку лучших зарубежных сериалов всех времен? Семейные сериалы смотреть онлайн бесплатно РІ хорошем качестве HD 720 без регистрации » Страница 9
Carisa Botero
| #
Com o aplicativo [leao](https://leao-88.com), você tem uma experiência de apostas sem igual. Baixe o app de maneira simples, instale e desfrute da interface otimizada, com navegação rápida e fluída, proporcionando a melhor jogabilidade a qualquer hora e lugar.
MarkNOlaf
| #
dark web market list https://drdarkfoxmarket.com – dark web market list
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://okkord.ru/
Jewell Guy
| #
Ao se registrar como novo usuário na [luck 2](https://luck-2.com), você não só tem acesso a uma das melhores plataformas de cassino online, mas também recebe um bônus de US$ 100 para começar a jogar. Aproveite essa chance de ouro para aumentar suas chances de ganhar nas slots e outros jogos de cassino populares.
FNDaviditabe
| #
darkmarket https://firstdarkmarket.com/ – darknet marketplace
WilliamIRrutty
| #
darknet market list https://asap-market-onion.com/ – dark web drug marketplace
Debera Kirksey
| #
Bônus de US$ 100 para novos usuários: Como se registrar na [john bet](https://john-bet.com)!
prodvijenie saitov v moskve_dcOa
| #
продвижение веб сайтов москва продвижение веб сайтов москва .
MatthewsOf
| #
Зарубежные сериалы – это отличный способ подтянуть иностранный язык Криминальные сериалы смотреть онлайн бесплатно в хорошем качестве HD без регистрации » Страница 25
Kxyufaita
| #
darknet market https://cyberdarkmarkets.com/ – darknet drugs
CharlesSpolf
| #
Witamy w Slottica! Nasza platforma to harmonijne połączenie innowacji, różnorodności i najwyższych standardów bezpieczeństwa, zaprojektowane z myślą o wymagających graczach z Polski. Oferujemy nie tylko szeroki wybór gier – od dynamicznych automatów, przez strategiczne gry stołowe, po ekskluzywne sesje z live dealerami – ale także kompleksowe rozwiązania dostosowane do Twoich preferencji. Dzięki zaawansowanej technologii mobilnej, ciesz się płynnym dostępem do ulubionych rozgrywek na dowolnym urządzeniu, gdziekolwiek jesteś. Slottica Vladimir współpracuje wyłącznie z uznanymi dostawcami oprogramowania, gwarantując najwyższą jakość grafiki, uczciwość mechaniki i regularne aktualizacje biblioteki gier.
Volodyanaf
| #
darkmarket link https://darkmarketpremium.com/ – darkmarket 2025
lifeautobodymailm
| #
Experience the convergence of artistry and technology at Life Auto Body. We are proficient in revitalizing your vehicle into a unique gem . Our proficient team provides exceptional auto tuning packages . Feel the rush of driving a perfectly tuned car crafted to your taste. Visit us at https://lifeautobody.com/ and start your car’s transformation journey with us!
lkjhCype
| #
http://electrotrade.biz/images/pages/?filmu_pro_samoletu___zahvatuvaushiy_mir_v_nebe.html скачать фильм на ноутбук без торрента
MarkNOlaf
| #
dark web market urls https://cannahomedarknetdrugstore.com/ – darknet markets 2025
MatthewsOf
| #
Лучшие сериалы – это те, которые захватывают с первых серий Жуковский (фильм 1950) смотреть онлайн бесплатно в хорошем качестве
MatthewsOf
| #
Зарубежные сериалы позволяют увидеть другие культуры и традиции Казнить нельзя помиловать (сериал 2017, НТВ) 1-12 серия смотреть онлайн бесплатно в хорошем качестве
Markcen
| #
Хотите сдать квартиру в аренду? Читайте проверенные советы https://privateconstruction.ru/
MatthewsOf
| #
Зарубежные сериалы бывают настолько захватывающими, что невозможно оторваться Один на всех 1, 2 сезон (сериал 2012, Россия) смотреть онлайн все серии подряд бесплатно в хорошем качестве
FNDaviditabe
| #
darkmarkets https://onion-dark-market.shop/ – darknet markets onion address
Terencesam
| #
Игровые автоматы на деньги реальные Casino top
MetaMask Download
| #
MetaMask Chrome is essential for crypto users. It simplifies transactions, provides robust security, and integrates perfectly with DeFi.
WilliamIRrutty
| #
darknet market links https://asap-market-onion.com/ – darknet market lists
Kxyufaita
| #
dark web market links https://cannahome-darkmarket-online.com/ – dark websites
Volodyanaf
| #
darknet market links https://darkfoxdarkweb.com/ – darkmarket url
Blue60
| #
Сравнительный анализ бизнеса: https://www.tandem.edu.co/presentacion-the-space/
Devin Clampitt
| #
A [bet7](https://bet7-88.com) oferece uma oferta imperdível para novos jogadores! Ao se registrar no site, você recebe US$ 100 para apostar em diversos jogos de cassino. Essa é uma excelente oportunidade para aproveitar ao máximo sua experiência de apostas online sem riscos iniciais.
MatthewsOf
| #
Где найти список лучших русских сериалов всех времен? HD военные сериалы смотреть онлайн бесплатно в хорошем качестве » Страница 7
MarkNOlaf
| #
darkmarket link https://cannahomedarknetdrugstore.com/ – darkmarket url
Andree Henifin
| #
A [pk55](https://pk55-88.com) é a escolha ideal para quem busca diversão e segurança. Com uma ampla variedade de jogos populares e um sistema justo, você pode apostar sem preocupações. Além disso, a plataforma oferece bônus exclusivos que garantem mais chances de vitória e recompensas para todos.
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://prudmarket.ru/
MatthewsOf
| #
Какие русские сериалы стоит посмотреть в 2024 году? Сквозь чёрное стекло фильм смотреть онлайн бесплатно в хорошем качестве на русском языке
Potolki_dex
| #
Хорошо сочетаются с разными стилями: классика, модерн, минимализм – https://potolki-v-moskve.ru/
FNDaviditabe
| #
darknet market https://kingdom-marketplace.com/ – darkmarket
Rosalia Verrone
| #
Ao se registrar na [8800 bet](https://8800-bet.com), você ganha US$ 100 de bônus! O processo é simples e o login é imediato. Depois de criar sua conta, você pode começar a jogar em jogos como roleta, blackjack e slots, tudo com a vantagem do bônus de boas-vindas. Registre-se já!
WilliamIRrutty
| #
darknet markets url https://kingdomdarkmarketonline.com/ – dark web link
AlfredoBlape
| #
Looking for high-quality slots to play? AbeBet Casino offers the best online slots for all players!
At AbeBet abe-casino.com/tr/games/demo/pragmatic_play_starlight_princess_1000, you’ll find slot hits from leading providers.
Register now to get newbie gifts.
Dive into a world of winnings with AbeBet!
MatthewsOf
| #
Где можно посмотреть новый сезон моего любимого зарубежного сериала? Пустыня (сериал 2019, НТВ) смотреть онлайн все серии подряд бесплатно в хорошем качестве
Timothyvop
| #
казино онлайн
Kxyufaita
| #
onion dark website https://cyberdarkmarkets.com/ – dark market url
Volodyanaf
| #
dark web drug marketplace https://darkode-market.com/ – darkmarket list
Jariornma
| #
Где заказать диплом по нужной специальности?
Наша компания предлагает быстро и выгодно приобрести диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, штампами, подписями. Документ способен пройти лубую проверку, даже при использовании специального оборудования. Решите свои задачи быстро с нашей компанией.
Приобрести диплом о высшем образовании kupitediplom.ru/kupit-attestati-za-9-13/
Lazravr
| #
Быстро и просто приобрести диплом ВУЗа!
Мы изготавливаем дипломы любых профессий по приятным тарифам— asxdiploman.com/kupit-diplom-s-zaneseniem-v-reestr-12/
Iariorqdb
| #
Заказать документ университета можно в нашей компании в столице. http://www.festivalcinepamplona.com/2020/10/29/hello-world
MatthewsOf
| #
Зарубежные сериалы в хорошем качестве – это просто сказка Урга: Территория любви (фильм 1991) смотреть онлайн бесплатно в хорошем качестве
MatthewsOf
| #
Русские исторические сериалы заслуживают внимания Так бывает (фильм 2007) смотреть онлайн бесплатно в хорошем качестве
lkjhCype
| #
http://arbaletspb.ru/img/pgs/?luchshie_filmu_dlya_prosmotra.html сколько будет 7 от 2000
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://pultmaster-pro.ru/
MarkNOlaf
| #
darknet site https://cannahomedarknetdrugstore.com/ – dark websites
CharlesPUSLY
| #
Официальный веб-сайт казино Рояль предлагает стильную обстановку для гемблеров. С его изысканным дизайном и элитным выбором игр, Рояль является превосходным местом для тех, кто обожает качественное азартное развлечение. Исследуйте широкий спектр игровых автоматов и настольных игр на основном веб-сайте royalcasino-slots.com/ru/.
Казино Рояль предоставляет высокое качество клиентов и поддержку. Внимательная команда поддержки в наличии круглосуточно, готова реагировать на любые возникающие вопросы и исправить проблемы, обеспечивая беспрерывную игру и удовлетворение.
MatthewsOf
| #
Русские сериалы в жанре драмы всегда вызывают сильные эмоции Солнце, море и любовь (сериал 2023, Домашний) смотреть онлайн все серии подряд бесплатно в хорошем качестве
DavidCooma
| #
прокат дорогого авто аренда машины адлер цены
1win_vgpt
| #
cod promoțional 1win 1win5001.ru .
Georgianne Triplette
| #
Na [bet 4](https://bet-4-br.com), você encontra uma vasta seleção de jogos justos e bônus exclusivos!
MatthewsOf
| #
Русские сериалы становятся все популярнее за рубежом НТВ сериалы смотреть онлайн новинки все серии подряд в хорошем качестве HD без регистрации » Страница 29
FNDaviditabe
| #
darknet sites https://onion-dark-market.shop/ – darknet drug links
KennethDrula
| #
Купить водительские права официально
mostbet_cgei
| #
скачать мостбет официальный сайт скачать мостбет официальный сайт .
WilliamIRrutty
| #
onion dark website https://asap-market-onion.com/ – darknet links
obzorcaree
| #
Подробнее в материале в блоге об обзорах электронной техники диджитал гаджет ссылка
Cazrmbj
| #
Приобрести диплом о высшем образовании поспособствуем. Часто задаваемые вопросы – diplomybox.com/voprosy-i-otvety
MatthewsOf
| #
Люблю зарубежные сериалы за атмосферу и крутых персонажей Помнить (фильм 2022) смотреть онлайн бесплатно в хорошем качестве на русском языке
mostbet_ygSi
| #
motsbet http://www.mostbet1002.com.kg .
Volodyanaf
| #
dark web market https://darkfoxdarkweb.com/ – darknet drug store
Kxyufaita
| #
dark web market list https://cannahomemarket.link/ – dark web link
Frankglync
| #
casebattle
MatthewsOf
| #
Лучшие зарубежные сериалы – это настоящие блокбастеры Сериалы 2019 года смотреть онлайн бесплатно, лучшие Сериалы 2019 » Страница 23
MarkNOlaf
| #
dark web market links https://dark-web-versus.com – darknet site
WolfStriker
| #
Практические советы для https://mejorsintlc.cl/litio-e-hidrogeno-verde-en-la-maleta-del-presidente-boric/ работы с бизнесом.
Williamvop
| #
На площадке представлен широкий ассортимент травматического оружия https://travmat-kupit.ru/
MatthewsOf
| #
Где найти подборку русских сериалов с высоким рейтингом? Движение вверх (фильм 2017) смотреть онлайн бесплатно в хорошем качестве на русском языке
MatthewsOf
| #
Русские сериалы про врачей стали очень популярны Нарушение правил 1 сезон 4 серия смотреть онлайн бесплатно в хорошем качестве на русском языке
FNDaviditabe
| #
dark web markets https://mydarkmarketlink.com/ – darknet websites
ThomasFette
| #
томми ли
WilliamIRrutty
| #
tor drug market https://kingdomdarkmarketonline.com/ – darknet markets onion
Cazraxz
| #
Здравствуйте!
Без ВУЗа очень непросто было продвигаться вверх по карьере. В наше время этот документ не дает гарантий, что получится получить хорошо оплачиваемую работу. Куда более важное значение имеют навыки и знания специалиста, а также его постоянный опыт. По этой причине решение о покупке диплома можно считать мудрым и рациональным. Заказать диплом любого университета milanoregola.copiny.com/question/details/id/1052647
MatthewsOf
| #
Зарубежные сериалы – это возможность окунуться в другой мир Волчий остров (фильм 2012) смотреть онлайн бесплатно в хорошем качестве
MatthewsOf
| #
Какие зарубежные сериалы стоит посмотреть в жанре фэнтези? HD триллеры смотреть онлайн бесплатно в хорошем качестве » Страница 3
Volodyanaf
| #
dark web market urls https://aasap-market.com/ – darknet drug market
Kxyufaita
| #
darknet markets links https://cyberdarkmarkets.com/ – darknet drug market
MatthewsOf
| #
Люблю сериалы, в которых много неожиданных поворотов Веселые Расплюевские дни (фильм 1966) смотреть онлайн бесплатно в хорошем качестве
1win_cypt
| #
pariuri sportive moldova http://www.1win5001.ru .
MatthewsOf
| #
Люблю сериалы с историческим сюжетом, особенно русские Платье цвета моря (сериал 2023, Россия) смотреть онлайн бесплатно все серии подряд в хорошем качестве
mostbet_zyei
| #
mostbet chrono mostbet1003.com.kg .
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://repinskieozera.ru/
MarkNOlaf
| #
darknet markets https://dark-web-versus.com – dark web marketplaces
Jamesfrapy
| #
армада казино 2025
mostbet_vySi
| #
мостбет скачать казино http://mostbet1002.com.kg/ .
MatthewsOf
| #
Лучшие русские сериалы – это те, которые заставляют переживать за героев Внутри (турецкий сериал 2016) на русском языке смотреть онлайн все серии подряд бесплатно в хорошем качестве
FrankFiP
| #
https://ramregion.ru
Donaldlaf
| #
darkmarket list dark market link
Pingrutty
| #
darknet market list dark web sites
FNDaviditabe
| #
darkmarket list https://onion-dark-market.shop/ – darkmarket
Toliknaf
| #
dark markets 2025 darknet marketplace
WilliamIRrutty
| #
darknet markets https://idarknetmarket.com/ – darkmarkets
Rabyitabe
| #
darknet markets onion dark website
Volodyanaf
| #
darknet sites https://darkmarketpremium.com/ – darknet drugs
Kxyufaita
| #
bitcoin dark web https://cannahome-darkmarket-online.com/ – dark web market links
MatthewsOf
| #
Русские сериалы в жанре драмы всегда вызывают сильные эмоции Чары любви (сериал 2024, Домашний) смотреть онлайн бесплатно все серии подряд в хорошем качестве
obzorcaree
| #
Подробнее в обозревательном журнале чек-ноутбук ссылка
MatthewsOf
| #
Лучшие зарубежные сериалы – это те, которые заставляют думать Случайная любовь (турецкий сериал 2021) на русском языке смотреть онлайн все серии подряд бесплатно в хорошем качестве
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://shatimtrade-kizlyar.ru/
MatthewsOf
| #
Где можно скачать сериалы в хорошем качестве? Хроники призрачного племени (фильм 2015) на русском языке смотреть онлайн бесплатно в хорошем качестве
lkjhCype
| #
http://monetoss.ru/news/molodezghnue_serialu__pochemu_ih_tak_lubyat_.html тнт телепрограмма на сегодня тольятти
MarkNOlaf
| #
darknet market list https://kingdom-darkmarketplace.com – darknet site
DonDonfaita
| #
darknet market lists darknet market list
MatthewsOf
| #
Люблю зарубежные сериалы за оригинальные сценарии и качественную съемку Жемчужная свадьба 1 сезон 4 серия смотреть онлайн бесплатно в хорошем качестве на русском языке
Pingrutty
| #
dark web markets dark websites
MatthewsOf
| #
Какой зарубежный сериал стоит посмотреть на выходных? Спрячь меня (турецкий сериал 2023, Домашний) на русском языке смотреть онлайн все серии подряд бесплатно в хорошем качестве
Fang2203
| #
Хочу понять в https://epiczo.com/?p=254 бизнесе. Куда зайти?
MatthewsOf
| #
Где можно смотреть зарубежные сериалы бесплатно и без регистрации? Скоряк 2 сезон 30 серия смотреть онлайн бесплатно в хорошем качестве на русском языке
Donaldlaf
| #
dark market list darknet markets
FNDaviditabe
| #
darknet site https://mydarkmarketlink.com/ – dark market onion
Toliknaf
| #
darknet markets dark web markets
WilliamIRrutty
| #
darknet market list https://idarknetmarket.com/ – dark market 2025
MatthewsOf
| #
Какие русские сериалы можно посоветовать для просмотра? Семья Зацепиных (фильм 1977) смотреть онлайн бесплатно все серии подряд в хорошем качестве
Volodyanaf
| #
dark web drug marketplace https://darkfoxdarkweb.com/ – darknet drug market
Rabyitabe
| #
dark web drug marketplace darknet drugs
Terencesam
| #
Игровые автоматы с бонусом за регистрацию без депозита no deposit casino
Kxyufaita
| #
darknet markets https://cannahomemarket.link/ – darknet drug store
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://sibotdelka.ru/
MatthewsOf
| #
Зарубежные сериалы про детективов всегда на высоте Не бойся, я с тобой! (фильм 1981) смотреть онлайн бесплатно в хорошем качестве на русском языке
JasonIsole
| #
https://textme.work/
MarkNOlaf
| #
dark market list https://cannahomedarknetdrugstore.com/ – darkmarket list
Pingrutty
| #
darknet markets onion address dark web link
MatthewsOf
| #
Лучшие русские сериалы – это те, которые вызывают бурю эмоций Комедии смотреть онлайн бесплатно в хорошем качестве HD без регистрации » Страница 144
mikrozaim
| #
Микрозайм онлайн на карту без проверок срочно микрозайм онлайн на карту без проверок срочно
MatthewsOf
| #
Какие зарубежные сериалы можно посмотреть в жанре фантастики? Смотреть Сериалы 2015 онлайн бесплатно в HD качестве » Страница 7
GeorgeTom
| #
Source:
– badmintonplayer.ru
DonDonfaita
| #
darknet drug market dark web drug marketplace
vzyatzaim
| #
Займы интернет оформление займа онлайн
avtoplastics
| #
онлайн-магазин автозапчастей https://avtoplastics.ru
MatthewsOf
| #
Зарубежные сериалы – это возможность окунуться в другой мир Охотничий (фильм 2010) на русском языке смотреть онлайн бесплатно в хорошем качестве
digitalcaree
| #
Подробнее в обозревательном журнале new digital tech здесь
MatthewsOf
| #
Зарубежные сериалы – это всегда высокий уровень съемок и крутые спецэффекты Ангел-полицейский (аниме 1989) на русском языке смотреть онлайн все серии подряд бесплатно в хорошем качестве
RicardoDitle
| #
Glory Casino
FNDaviditabe
| #
dark web markets https://firstdarkmarket.com/ – dark markets 2025
Donaldlaf
| #
dark markets darknet markets onion
WilliamIRrutty
| #
darknet markets onion address https://darknetmarketsonion.shop/ – darknet markets
MatthewsOf
| #
Смотреть сериалы в хорошем качестве – настоящее удовольствие Сериалы 2023 года смотреть онлайн бесплатно, лучшие фильмы 2023, в хорошем качестве » Страница 35
Toliknaf
| #
dark market link dark market link
Volodyanaf
| #
darknet markets https://darkmarketpremium.com/ – darkmarket
Kxyufaita
| #
dark websites https://cannahome-darkmarket-online.com/ – darknet drugs
Rabyitabe
| #
darkmarket 2025 dark market 2025
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://snegirieko.com/
MatthewsOf
| #
Ищу зарубежный сериал, который можно смотреть без остановки Дом с чёрными котами (сериал 2018) смотреть онлайн все серии подряд бесплатно в хорошем качестве
Pingrutty
| #
dark markets 2025 darknet sites
MarkNOlaf
| #
tor drug market https://kingdom-darkmarketplace.com – dark web market list
DonDonfaita
| #
darknet markets darknet markets
Andrewwhone
| #
pokerdom
durmitor
| #
prirodni biser Durmitor park Jedinstveni planinski pejzazi, bistra jezera, kanjoni i guste sume. Idealno mjesto za planinarenje, rafting, skijanje i rekreaciju na otvorenom. Otkrijte nacionalni park pod zastitom UNESCO-a!
nuevoscasinosonlineespana
| #
codigo promocional de olimpo bet nuevoscasinosonlineespana.com
FNDaviditabe
| #
darknet markets https://mydarkmarketlink.com/ – darknet drug links
Andrewwhone
| #
joycasino бонус код
Donaldlaf
| #
dark market url darknet links
WilliamIRrutty
| #
darknet market list https://darknetmarketsonion.shop/ – onion dark website
Volodyanaf
| #
darkmarkets https://darkfoxdarkweb.com/ – dark web market
MatthewsOf
| #
Русские сериалы про реальную жизнь всегда интересны Мелодрамы смотреть онлайн бесплатно в хорошем качестве HD » Страница 34
Toliknaf
| #
darknet market lists dark markets 2025
mtsnovosibirsksig
| #
провайдер мтс
https://digitalbarker.com/employer/gertrude2406
мтс интернет
Kxyufaita
| #
darkmarket 2025 https://kingdomdarknetdrugstore.com/ – darknet markets
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://so35.ru/
Pingrutty
| #
darknet markets dark market url
Blade7928
| #
https://dmpublicidad.com.ar/974-2/ Пример из практики: Бизнес.
Trefujx
| #
Заказать документ университета вы сможете в нашей компании. diplom-club.com/kupit-diplom-krasnoyarsk-4
MatthewsOf
| #
Всегда ищу лучшие русские сериалы, чтобы насладиться интересными историями Татьянина ночь (сериал 2014) смотреть онлайн все серии подряд бесплатно в хорошем качестве
MarkNOlaf
| #
tor drug market https://drdarkfoxmarket.com – darknet market links
Tamojennii predstavitel Moskva_vmki
| #
таможенный брокер импорт https://tamozhennyj-predstavitel-moskva12.ru/ .
Tamojennii broker Moskva_wcKr
| #
компания таможенный брокер компания таможенный брокер .
prodvijenie saitov v moskve_oxOa
| #
продвижение сайта онлайн продвижение сайта онлайн .
DonDonfaita
| #
darkmarket link darkmarket url
1win_omPn
| #
win 1 http://cah.forum24.ru/?1-19-0-00000716-000-0-0-1741702224/ .
Sazrweg
| #
купить диплом петербург одно
FNDaviditabe
| #
bitcoin dark web https://onion-dark-market.shop/ – darkmarket link
WilliamIRrutty
| #
onion dark website https://darknetmarketsonion.shop/ – darkmarket list
mostbet_dppt
| #
скачат мостбет http://dubna.myqip.ru/?1-18-0-00000145-000-0-0-1741708632 .
Volodyanaf
| #
dark web market https://darkode-market.com/ – darkmarket 2025
Donaldlaf
| #
dark web market links dark web sites
Pingrutty
| #
darknet markets links darknet drug market
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://sochi-park-dom.ru/
Toliknaf
| #
darknet market darknet market list
MatthewsOf
| #
Лучшие русские сериалы – это те, которые заставляют задуматься Гордость мира боевых искусств (дорама 2023) на русском языке смотреть онлайн все серии подряд бесплатно в хорошем качестве
Kxyufaita
| #
tor drug market https://kingdomdarknetdrugstore.com/ – dark web sites
proektoroffcaree
| #
Подробнее в обозревательном журнале proektorOFF на сайте
Rabyitabe
| #
dark web markets dark websites
1win_eaKl
| #
1win.online https://aktivnoe.forum24.ru/?1-2-0-00000100-000-0-0-1741701286 .
MarkNOlaf
| #
dark market https://kingdomdarkmarketplace.com – darknet links
Mazrtdv
| #
Где купить диплом специалиста?
Мы оказываем услуги по изготовлению и продаже документов об окончании любых университетов РФ. Документы производят на оригинальных бланках. lightsdemons.phorum.pl/viewtopic.php?p=70808#70808
DonDonfaita
| #
dark web link dark web market urls
Pingrutty
| #
darknet markets onion darkmarkets
FNDaviditabe
| #
darkmarket https://onion-dark-market.shop/ – onion dark website
Volodyanaf
| #
darknet markets url https://darkmarketpremium.com/ – darknet markets links
mtsnovosibirsksig
| #
мтс подключение
https://www.listandrelax.com/profile/johnniechaves
мтс подключить
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://technovoda.ru/
Donaldlaf
| #
dark market best darknet markets
1win_wcPn
| #
1win https://cah.forum24.ru/?1-19-0-00000716-000-0-0-1741702224/ .
Kxyufaita
| #
dark web market urls https://cannahomemarket.link/ – darknet markets onion
Toliknaf
| #
tor drug market darkmarket list
mostbet_bzpt
| #
мостбет промокод http://dubna.myqip.ru/?1-18-0-00000145-000-0-0-1741708632/ .
Rabyitabe
| #
dark market darknet market list
МФО новые
| #
Когда экстренно нужны деньги, а банки не одобряют, альтернатива есть — быстрые микрозаймы. Теперь взять кредит может каждый, ключевое условие — паспорт. Наша группа экспертов собрала список из десятков малоизвестные займы на карту онлайн которые работают официально и выдают суммы до 30 тысяч гарантированно.
Даже если вам отказывали в финансирование, в этом списке шанс почти 100%! Тарифы выгодные, подписание договора моментальное. Без звонков, изучения кредитной истории и справок. Такие займы — лучшее решение, когда средства нужны здесь и сейчас.
MarkNOlaf
| #
darknet websites https://dark-web-versus.com – darkmarket link
Pingrutty
| #
dark web sites dark markets 2025
Terencesam
| #
Игровые автоматы играть на деньги 1000 рублей за регистрацию вывод сразу
DonDonfaita
| #
dark web market list dark web market urls
FNDaviditabe
| #
dark web marketplaces https://firstdarkmarket.com/ – darknet links
Volodyanaf
| #
dark market 2025 https://darkode-market.com/ – tor drug market
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://territoria-we-stars.ru/
WilliamIRrutty
| #
darknet marketplace https://darknetmarketsonion.shop/ – darkmarket list
МФО новые
| #
Когда срочно нужны средства, а финансовые организации отказывают, решение есть — быстрые микрозаймы. Теперь оформить займ может все, главное условие — паспорт. Наша специалисты собрала базу из более 20 самые новые займы которые легально действуют и предоставляют небольшие кредиты гарантированно.
Если раньше вам отказывали в кредит, в этом списке шанс максимальный! Тарифы низкие, подписание договора моментальное. Никаких звонков, проверок и справок. Такие займы — оптимальный вариант, когда финансы нужны здесь и сейчас.
Donaldlaf
| #
dark websites darkmarket link
1win_mqKl
| #
1wln http://www.aktivnoe.forum24.ru/?1-2-0-00000100-000-0-0-1741701286 .
Kxyufaita
| #
darknet sites https://kingdomdarknetdrugstore.com/ – dark web markets
Michaelabaks
| #
Это лучший способ вернуть себе полноценную улыбку без ущерба для соседних зубов https://dentalaesthetics.ru/
Sazrzbr
| #
Приветствую!
Приобрести диплом ВУЗа по доступной стоимости можно, обращаясь к проверенной специализированной компании. Купить диплом: diplomt-v-chelyabinske.ru/kupit-diplom-kursk-5/
Toliknaf
| #
bitcoin dark web darknet drugs
MatthewsOf
| #
Всегда жду выхода новых сезонов любимых зарубежных сериалов Драмы смотреть онлайн бесплатно в хорошем качестве HD без регистрации » Страница 20
Rabyitabe
| #
tor drug market darknet markets 2025
Lesha Hipple
| #
Venha para a [f12bet](https://f12–bet.com) e aproveite os jogos de cassino justos com bônus imperdíveis!
MarkNOlaf
| #
dark market onion https://kingdom-darkmarketplace.com – darknet market
Lesha Hipple
| #
A [bet7](https://bet-7-br.com) oferece um bônus de US$ 100 para novos jogadores assim que se registram no site. Não perca tempo e aproveite essa chance de começar a sua experiência no cassino com um bônus que vai dar aquele empurrão inicial nas suas apostas. Faça seu cadastro agora mesmo!
Pingrutty
| #
darknet market list bitcoin dark web
mtsnovosibirsksig
| #
мтс тарифы на интернет
https://empleo.infosernt.com/employer/shanna1183/
мтс тв новосибирск
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://texnotect.ru/
Volodyanaf
| #
darkmarket link https://darkfoxdarkweb.com/ – dark market 2025
FNDaviditabe
| #
darkmarket link https://onion-dark-market.shop/ – dark web market links
DonDonfaita
| #
darknet markets links darknet drug store
WilliamIRrutty
| #
darknet markets links https://kingdomdarkmarketonline.com/ – dark market onion
Michaelabaks
| #
Имплантация позволяет избавиться от неудобств, связанных с ношением съемных протезов: этапы имплантации зубов
MatthewsOf
| #
Зарубежные сериалы по книгам часто бывают очень интересными Эластико смотреть онлайн бесплатно в хорошем качестве на русском языке
RedZ3
| #
Где смотреть статистику о бизнесе? https://shop.platinumwellness.net/hello-world/
MatthewsOf
| #
Русские сериалы про криминал всегда интересные и захватывающие Одноклассники.ru: НаCLICKай удачу фильм смотреть онлайн бесплатно в хорошем качестве на русском языке
Lazrmfg
| #
Диплом ВУЗа России!
Без ВУЗа достаточно сложно было продвигаться по карьерной лестнице. Именно из-за этого решение о покупке диплома стоит считать мудрым и рациональным. Приобрести диплом института deves.flybb.ru/viewtopic.php?f=2&t=1168
Kxyufaita
| #
darknet websites https://cannahomemarket.link/ – dark web drug marketplace
MatthewsOf
| #
Зарубежные сериалы по книгам часто бывают очень интересными Бродяга (дорама 2019) на русском языке смотреть онлайн бесплатно все серии подряд в хорошем качестве
Toliknaf
| #
dark web marketplaces darknet links
MatthewsOf
| #
Смотреть сериалы в хорошем качестве – настоящее удовольствие Жила-была девочка фильм смотреть онлайн бесплатно в хорошем качестве на русском языке
Pingrutty
| #
darkmarkets darkmarket list
Rabyitabe
| #
dark web marketplaces darknet market
MarkNOlaf
| #
bitcoin dark web https://drdarkfoxmarket.com – darknet markets
MatthewsOf
| #
Какие русские сериалы стоит посмотреть в 2024 году? Одноклассники.ru: НаCLICKай удачу фильм смотреть онлайн бесплатно в хорошем качестве на русском языке
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://tolmi74.ru/
lkjhCype
| #
https://ourmind.ru//wp-content/news/filmu_pro_budushee__vzglyad_skvoz_prizmu_vremeni.html фильмы фул hd 1080 смотреть онлайн бесплатно
Volodyanaf
| #
darknet markets url https://darkode-market.com/ – dark web markets
MatthewsOf
| #
Где можно смотреть сериалы без регистрации и смс? Мелодрамы смотреть онлайн бесплатно в хорошем качестве HD » Страница 51
FNDaviditabe
| #
darkmarket link https://onion-dark-market.shop/ – dark market url
WilliamIRrutty
| #
dark websites https://darknetmarketsonion.shop/ – dark markets
DonDonfaita
| #
dark web drug marketplace dark web market list
Diplomi_bwKt
| #
Приобрести диплом любого университета!
Мы можем предложить документы институтов, которые расположены на территории всей РФ.
Превосходства наших дипломов:
• используем настоящие бланки “Гознака”;
• необходимые подписи руководства;
• мокрые печати учебного заведения;
• специальные водяные знаки, нити и прочие степени защиты;
• идеальное заполнение и оформление – ошибок не бывает;
• любая проверка документов.
magazin-diplomov.ru/kupit-diplom-v-bryanske-3
Kxyufaita
| #
dark market link https://cannahomemarket.link/ – dark web link
Donaldlaf
| #
best darknet markets darknet drug links
Pingrutty
| #
dark web sites darknet market
Toliknaf
| #
dark markets dark web market urls
ufc_ar
| #
everything about the world https://t.me/ufc_ar of UFC in Arabic! Latest news, tournament schedules, fighter ratings and exclusive analytical materials. Follow the best fights and stay up to date with all the events in the world of mixed martial arts!
MarkNOlaf
| #
dark markets 2025 https://kingdom-darkmarketplace.com – darknet drug links
Rabyitabe
| #
darknet markets links darknet market
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://topkadastr-crimea.ru/
MasonBed
| #
steam desktop authenticator github
Volodyanaf
| #
darknet market links https://aasap-market.com/ – dark web link
FNDaviditabe
| #
dark web link https://darkmarketdarkfox.com/ – onion dark website
Jamesbop
| #
Looking for best online casino games? MasalBet is the place to find favorite games from renowned providers.
At https://denizliotoekspertiz.com, you’ll find newbie gifts and a user-friendly interface.
Sign up now and get amazing bonuses.
Play true excitement with Masal Bet!
WilliamIRrutty
| #
darknet market list https://kingdommarketdarknet.com/ – dark web marketplaces
Jamesbop
| #
Игровая платформа 7k — это современная платформа для игр с ставками с огромным выбором развлечений на любой вкус. Здесь можно найти лучшие слот-машины, настольные игры и реальные игры с дилерами с опытными дилерами. Интерфейс пространный и простой в навигации, что делает игру удобной и увлекательной. На 7k740.casino предусмотрены щедрые бонусы для игроков. В целом, ресурс предоставляет отличный опыт для каждого игрока.
DonDonfaita
| #
dark web drug marketplace darknet links
JosephElari
| #
https://msk.sweet-escort.co/
Pingrutty
| #
dark market 2025 dark market link
Pioneer4200
| #
Пытаюсь разобраться в http://vitanuova.ru/u-severnoj-korei-est-svoj-planshet/ бизнесе. Что посоветуете?
Kxyufaita
| #
darknet market list https://darkfoxmarketplace.com/ – darknet markets onion address
mostbet_noKr
| #
мосбет https://www.aktivnoe.forum24.ru/?1-8-0-00000260-000-0-0-1741701879 .
lkjhCype
| #
https://yarkompany.ru/language/pages/?volshebnue_skazki___okna_v_mir_chudes.html фильмы с высоким рейтингом зарубежные смотреть онлайн
Donaldlaf
| #
darknet markets onion darknet market lists
1win_gsPn
| #
1 win официальный https://aktivnoe.forum24.ru/?1-8-0-00000259-000-0-0-1741701621/ .
Mazrpro
| #
Добрый день!
Где заказать диплом по актуальной специальности?
Мы изготавливаем дипломы любых профессий по приятным тарифам. Стоимость может зависеть от определенной специальности, года выпуска и ВУЗа. Стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас очень важно, чтобы дипломы были доступны для большого количества наших граждан.
Покупка документа, подтверждающего окончание института, – это выгодное решение. Просто посчитайте, сколько понадобится потратить средств на ежемесячную оплату 5 лет обучения, на аренду квартиры (если учащийся из другого города), на проезд до ВУЗа и многие другие издержки. Получится приличная сумма, которая превышает цены на наши документы. А ведь все это время можно уже с успехом работать, развивая собственные навыки на практике.
Готовый диплом с приложением отвечает стандартам, неотличим от оригинала. Не откладывайте свои мечты и цели на потом, реализуйте их с нашей компанией – отправьте простую заявку на изготовление документа сегодня!
Диплом о высшем образовании – легко! diplomanruss.com/
Toliknaf
| #
darknet links darknet markets url
MarkNOlaf
| #
darknet markets https://cannahomedarknetdrugstore.com/ – darknet site
DonaldTob
| #
Сертификат происхождения СТ-1 подтверждает, что товар был произведен в определенной стране, что важно для таможенного оформления и применения тарифных преференций. Протокол испытания, в свою очередь, документирует результаты лабораторных исследований продукции, подтверждая соответствие определенным стандартам качества и безопасности. Сертификат соответствия на продукцию удостоверяет, что товар соответствует требованиям технических регламентов или национальных стандартов. Свидетельство о государственной регистрации продукции требуется для товаров, подлежащих санитарно-эпидемиологическому надзору, и подтверждает их безопасность для здоровья человека. Декларация ТР ТС – это документ, которым производитель или импортер подтверждает соответствие продукции требованиям технических регламентов Таможенного союза. Нотификация ФСБ требуется для ввоза или вывоза продукции, содержащей шифровальные (криптографические) средства. Декларация Минсвязи, в свою очередь, необходима для оборудования связи, подтверждая его соответствие установленным требованиям в сфере связи и телекоммуникаций. Все эти документы играют важную роль в обеспечении качества, безопасности и законности продукции, обращающейся на рынке. сертификат происхождения СТ-1
Markcen
| #
Покупка квартиры без рисков? Полезные статьи и советы — https://uk-arbat.ru/
Rabyitabe
| #
darknet drug store darknet drug store
ThomasMaw
| #
иногда лучше доверить seo профессионалу, чтобы избежать ошибок https://seo-tver.ru/
MasonBed
| #
скачать steam desktop authenticator github
mostbet_hnKa
| #
mostbet промокод mostbet промокод .
Volodyanaf
| #
best darknet markets https://darkmarketpremium.com/ – tor drug market
Danielgen
| #
ProxyLib for Startups
FNDaviditabe
| #
darknet site https://onion-dark-market.shop/ – tor drug market
WilliamIRrutty
| #
darkmarkets https://idarknetmarket.com/ – darknet websites
Pingrutty
| #
darknet markets onion address darkmarket url
kiprus
| #
аренда авто в сиде прокат машин сочи без водителя
Terencesam
| #
Бонусы без отыгрыша за регистрацию фриспины без депозита с выводом за регистрацию
DonDonfaita
| #
bitcoin dark web dark market onion
Kxyufaita
| #
darknet market links https://cannahomemarket.link/ – onion dark website
DenverPek
| #
“Witryna pia, 12 sierpnia 2018 to katastrofalna klapa! Laduje sie z opoznieniem, pliki zawsze znikaja, a pomoc kontaktuje sie bardzo wolno.
Interfejs jest nieintuicyjny, funkcje uruchamiaja sie z bledami, a produkt obrazow pozostawia wiele do zyczenia.
Zarejestrowalem sie na konto premium, kwota zostaly zabrane natychmiast, ale dostep do przywilejow nie otworzyl sie. To po prostu oszustwo!
Nie polecam przyjaciolom, zmarnujesz zasoby i oszczednosci.”
Donaldlaf
| #
darkmarkets darkmarkets
Toliknaf
| #
dark market onion dark web marketplaces
MarkNOlaf
| #
dark web sites https://kingdom-darkmarketplace.com – dark web link
Zulma Bywater
| #
https://api.lioleo.edu.vn/public/images/course/4.png.php?id=link-pg-bet
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://vernadskogo-loft.ru/
lkjhCype
| #
https://school33-perm.ru/media/pgs/?hitu_amediateki.html фильмы с высокими рейтингами смотреть онлайн бесплатно в хорошем качестве
MasonBed
| #
steam authenticator
mostbet_jnKr
| #
мостбет скачать мостбет скачать .
Billy Ishak
| #
https://lioleo.edu.vn/creatorcourse02/4.png.php?id=joker-bet-21
Rabyitabe
| #
darknet markets 2025 darknet links
Coreen Giarusso
| #
https://lioleo.edu.vn/daily01-huyen/4.png.php?id=bet-roulette
1win_gjPn
| #
1win бк https://aktivnoe.forum24.ru/?1-8-0-00000259-000-0-0-1741701621/ .
Volodyanaf
| #
darknet drug market https://darkode-market.com/ – darknet drug market
Leoma Witthuhn
| #
https://lioleo.edu.vn/daily01-huyen/4.png.php?id=bet-city
Pingrutty
| #
darknet markets onion darknet drug links
FNDaviditabe
| #
darknet markets onion https://firstdarkmarket.com/ – bitcoin dark web
mtsnovosibirsksig
| #
мтс подключить новосибирск
https://smlord.com/employer/efrain2784/
мтс подключение новосибирск
Prodvizhen_mror
| #
Как продвигать сайт успешно, изучите.
Продвижение сайтов: от теории к практике, знания.
Узнайте, как продвигать сайт, которые повысит.
Будущее онлайн-продвижения, которые изменят ваш бизнес.
Углубляемся в продвижение сайтов, доступные каждому.
Продвижение сайтов в 2025 году, которые нужно освоить.
Лучшие агентства по SEO, на которые можно положиться.
14 ошибок при продвижении сайтов, чтобы не потерять трафик.
Доступные методы SEO, без лишних затрат.
Топовые инструменты для анализа, что упростят вашу работу.
Как анализировать успех продвижения сайта, чтобы добиться результата.
Как контент влияет на трафик, не забывайте.
Успех в локальном SEO, стратегии, которые работают.
Психология пользователей и SEO, приемы, которые сработают.
Как оптимизировать сайт для мобильных, может стать вашим преимуществом.
Как выбрать метод продвижения, на основе фактов.
Значение ссылочного продвижения, чтобы повысить авторитет.
Ежегодные тренды в SEO, изучите подробнее.
Сила SMM в SEO, вовлекайте пользователей.
Как правильно оптимизировать, используйте для продвижения.
продвижение сайта в гугл продвижение сайта в гугл .
WilliamIRrutty
| #
darknet markets onion address https://asap-market-onion.com/ – dark web market list
Tuyet Measheaw
| #
https://lioleo.edu.vn/christmas-section/4.png.php?id=bet-365
Jeannie Forss
| #
https://api.lioleo.edu.vn/public/images/course/4.png.php?id=bet-1000
pinco kazino _jjmt
| #
pinko casino pinko casino .
mostbet_peKa
| #
мостбет скачать cah.forum24.ru/?1-19-0-00000715-000-0-0-1741702061 .
ufc-ar
| #
Follow UFC fights with https://t.me/s/ufc_ar full tournament schedule, fight results, fighter ratings and analytics. Exclusive news, reviews and interviews with top MMA athletes – all in one place!
DonDonfaita
| #
darknet drug store darknet market links
pinco kazino _xzSn
| #
pinko casino pinko casino .
casino-reyting
| #
Рейтинг казино лицензионные https://t.me/casino_list_reyting/
Kxyufaita
| #
darknet links https://kingdomdarknetdrugstore.com/ – dark market url
DanielRup
| #
Уже много лет мы предоставляем услуги по ремонту и обслуживанию автомобилей Citroen, обеспечивая надежность и безопасность вашего автомобиля. Наши специалисты проводят диагностику, замену запчастей и техническое обслуживание как для владельцев легковых автомобилей, так и для корпоративных клиентов, гарантируя высокий уровень сервиса и индивидуальный подход к каждому автомобилю https://citroen-avtoservise.ru/
JamesPat
| #
задумываетесь об улучшении позиций вашего сайта в поисковой выдаче? возможно, вам будет полезен этот материал https://1st-seo.ru/
Phantom_Warden
| #
Лучшие инструменты для работы с бизнесом: http://www.pureatz.com/recipes/penne-with-shrimp-and-asparagus/
Donaldlaf
| #
darknet drug market darkmarkets
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://worldstone33.ru/
MarkNOlaf
| #
dark markets 2025 https://kingdomdarkmarketplace.com – darkmarket
Toliknaf
| #
darknet drug links dark market
Davidpeark
| #
Zajrzalem na strone 1496 bridge convention, i bez owijania w bawelne, calkowicie zawiedziony. Oprawa wizualna wyglada przestarzale, czesto pojawiaja sie problemy podczas otwierania, co utrudnia sprawnym dzialaniu. Nie wspominajac o tym, ze prezentacja informacji nie jest najlepsza. Support praktycznie nie istnieje, co sprawia, ze sytuacja staje sie jeszcze bardziej uciazliwa. Dostepne gry, jesli w ogole mozna je tak nazwac, sa calkowicie nieciekawe i nawet czesto sie zawieszaja. Nikomu tracic swojego czasu na te strone, jesli chcecie przyjemnej gry.
Lavina Mantano
| #
https://lioleo.edu.vn/app-dang-ky-thanh-cong/4.png.php?id=bet-slot-apk
Pingrutty
| #
dark web drug marketplace dark markets
Rabyitabe
| #
dark web marketplaces dark market onion
Volodyanaf
| #
darknet sites https://aasap-market.com/ – darknet markets onion
Malorie Parrot
| #
https://api.lioleo.edu.vn/public/images/course/4.png.php?id=bet-pemda
FNDaviditabe
| #
darknet links https://darkmarketdarkfox.com/ – darknet market links
WilliamIRrutty
| #
darkmarket url https://darknetmarketsonion.shop/ – darknet market
Selina Wasielewski
| #
https://lioleo.edu.vn/creatorcourse01/4.png.php?id=guci-bet
DonDonfaita
| #
best darknet markets darkmarkets
Kxyufaita
| #
darknet drug links https://cyberdarkmarkets.com/ – darknet drug store
Lazroqw
| #
Где заказать диплом по необходимой специальности?
Приобрести диплом института по невысокой цене возможно, обращаясь к проверенной специализированной фирме.: magazin-diplomov.ru
lkjhCype
| #
https://allboiler.ru/news/poznavatelnoe_kino__uchimsya__razvivaemsya_i_vdohnovlyaemsya.html fc zenit ru официальный сайт
WilliamDix
| #
Мы изготавливаем дипломы любой профессии по приятным тарифам. Дипломы производятся на подлинных бланках государственного образца Заказать диплом о высшем образовании diplomt-tver69.ru
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://komproekt-spb.ru/
Donaldlaf
| #
dark market onion darknet markets url
MarkNOlaf
| #
darknet marketplace https://dark-web-versus.com – dark market 2025
Pingrutty
| #
dark markets dark web market
Toliknaf
| #
darknet drugs dark web market
mtsnovosibirsksig
| #
провайдер мтс
https://bcde.ru/employer/autumn8982/
мтс подключить
Volodyanaf
| #
darknet markets onion address https://darkode-market.com/ – bitcoin dark web
Rabyitabe
| #
best darknet markets darknet sites
FNDaviditabe
| #
darkmarket url https://darkmarketdarkfox.com/ – dark markets
WilliamIRrutty
| #
dark web link https://asap-market-onion.com/ – darknet links
бнанс Створити акаунт
| #
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Kxyufaita
| #
dark markets 2025 https://kingdomdarknetdrugstore.com/ – onion dark website
Cyndy Wadkins
| #
https://lioleo.edu.vn/app-dang-ky-thanh-cong/4.png.php?id=golf-bet
DonDonfaita
| #
tor drug market darknet marketplace
Mazrkun
| #
Где купить диплом по необходимой специальности?
Готовый диплом со всеми печатями и подписями 100% отвечает запросам и стандартам Министерства образования и науки, неотличим от оригинала. Не откладывайте свои мечты и задачи на потом, реализуйте их с нами – отправляйте быструю заявку на диплом уже сегодня! Диплом о среднем образовании – не проблема! diplom-top.ru/kupit-diplom-medrabotnika-bistro-i-nadezhno/
Pingrutty
| #
dark market list dark web market list
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://konsta-ovk.ru/
Lindsay Warlick
| #
https://lioleo.edu.vn/creatorcourse01/4.png.php?id=qq1221-bet
MarkNOlaf
| #
dark web markets https://drdarkfoxmarket.com – darknet drug links
Donaldlaf
| #
darknet drug store darkmarket list
lkjhCype
| #
https://clockfase.com/auth/elmn/?filmu_na_kinogo___luchshiy_vubor_dlya_lubiteley_kino.html скачать фильм на телефон без регистрации
Toliknaf
| #
darknet websites darkmarkets
Volodyanaf
| #
darkmarket url https://darkode-market.com/ – dark market link
Dark555
| #
Авторитетное мнение о бизнесе: https://mattaarquitectos.es/portfolio/avenida-reina-victoria-8-madrid/main-31/
Rabyitabe
| #
darknet markets links darknet markets onion
FNDaviditabe
| #
dark web market list https://onion-dark-market.shop/ – darkmarket url
WilliamIRrutty
| #
darknet market list https://asap-market-onion.com/ – best darknet markets
Sazrdev
| #
Очень часто случается так, что для продвижения по карьерной лестнице, требуется документ, который подтверждает наличие профильного образования. Где заказать диплом специалиста?
Купить документ института вы имеете возможность у нас. Мы предлагаем документы об окончании любых университетов РФ. bagira-school.com.ua/
Xazrojr
| #
Приобрести диплом о высшем образовании!
Мы изготавливаем дипломы любых профессий по приятным ценам. Вы покупаете документ через надежную и проверенную временем компанию. : diplomc-v-ufe.ru/kupit-diplom-veip-o-visshem-obrazovanii-na-blanke-goznak/
Terencesam
| #
Top casino с выводом 1000 рублей за регистрацию вывод сразу
Christian Fonteneau
| #
https://api.lioleo.edu.vn/public/images/skills/4.png.php?id=jet88-bet
Kxyufaita
| #
darknet site https://cannahome-darkmarket-online.com/ – dark market url
Pingrutty
| #
dark web market links dark web drug marketplace
Dnrtqro
| #
Где купить диплом специалиста?
Мы изготавливаем дипломы любой профессии по невысоким тарифам. Мы готовы предложить документы ВУЗов, расположенных в любом регионе Российской Федерации. Можно купить диплом за любой год, указав необходимую специальность и хорошие оценки за все дисциплины. Документы выпускаются на “правильной” бумаге высшего качества. Это дает возможности делать настоящие дипломы, не отличимые от оригинала. Документы будут заверены необходимыми печатями и подписями. Стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас важно, чтобы документы были доступны для большого количества граждан. kupitediplom0029.ru/kupit-diplom-rossiya-2-3
DonDonfaita
| #
dark web sites dark web sites
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://monsalvat.ru/
mtsnovosibirsksig
| #
провайдер мтс
https://cannabisjobs.solutions/companies/daniella5036/
мтс тарифы новосибирск
MarkNOlaf
| #
darknet markets onion https://cannahomedarknetdrugstore.com/ – darknet links
Sazrkkf
| #
Приобрести диплом о высшем образовании !
Покупка диплома любого ВУЗа России у нас является надежным делом, потому что документ будет заноситься в реестр. Печать выполняется на фирменных бланках ГОЗНАКа. Купить диплом об образовании arenadiplom24.online/vuzy/uralskij-gosudarstvennyj-universitet-putej-soobshcheniya
Donaldlaf
| #
dark web market darkmarket link
Volodyanaf
| #
darknet drug store https://darkmarketpremium.com/ – darkmarket
Toliknaf
| #
darknet links darknet drugs
Cazrrzs
| #
Приобрести диплом университета по невысокой цене вы сможете, обратившись к проверенной специализированной фирме. Мы можем предложить документы учебных заведений, расположенных в любом регионе РФ. diplomers.com/kak-kupit-diplom
GeorgeTom
| #
Source:
– eurekatomsk.ru
FNDaviditabe
| #
darknet site https://kingdom-marketplace.com/ – dark web market urls
BryanNeisM
| #
Играйте в Mostbet Casino mostbet spas extreme лицензированное онлайн-казино с огромным выбором игр! Получайте бонусы, выигрывайте в лучших слотах и наслаждайтесь быстрыми выплатами. Удобные способы пополнения счета!
Rabyitabe
| #
darkmarket url dark web markets
Diplomi_dnKt
| #
Приобрести диплом университета!
Мы готовы предложить документы университетов, расположенных на территории всей РФ.
diplom-onlinex.com/magistr-kupit-diplom-2
Sazrfll
| #
Заказать диплом университета по невысокой цене возможно, обращаясь к надежной специализированной компании. Мы оказываем услуги по производству и продаже документов об окончании любых университетов России. Приобрести диплом университета– diploma-groups24.ru/kupit-diplom-v-gorode/cherepovec.html
WilliamIRrutty
| #
dark web link https://kingdomdarkmarketonline.com/ – darknet market
Pingrutty
| #
dark web sites darknet market links
Anjelica Mac
| #
https://lioleo.edu.vn/app-dang-ky-thanh-cong/4.png.php?id=ppg-bet
Kxyufaita
| #
dark web market urls https://darkfoxmarketplace.com/ – darkmarket 2025
lkjhCype
| #
https://taktikiipraktiki.ru/news/serialu_pro_derevnu__prostota__iskrennost_i_osobaya_atmosfera.html смотреть фильм гримм все серии
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://chelstg.ru/
Niki Rhea
| #
https://lioleo.edu.vn/christmas-section/4.png.php?id=bet-hoki-77
Anthony_Inads
| #
накрутка лайков в ВК на пост бесплатно
DonDonfaita
| #
darknet markets darknet site
Jamesenved
| #
Автополив участка — идеальное решение для вашего сада и дачи! Наша ландшафтная студия предлагает профессиональный монтаж автоматической системы орошения, которая гарантирует эффективный полив и уход за вашим газоном и растениями. Забудьте о рутинном поливе! Мы проведем проектирование, установку и настройку оборудования под ваши нужды. С нашим сервисом вы сможете наслаждаться уютом загородного участка без забот. Обеспечьте своим растениям качественный уход с помощью автополива https://1landshaftniy-dizayn.ru/
Charlesnag
| #
Выбираем недвижимость в Киеве https://automat.kiev.ua как оценить район, новостройки и вторичный рынок? Анализ цен, проверка застройщика, юридическая безопасность сделки. Полезные советы для покупки квартиры без ошибок и переплат!
Anthony_Inads
| #
накрутка лайков в ВК онлайн бесплатно
Jamespeege
| #
Строительный онлайн журнал https://kero.com.ua ваш источник актуальной информации о строительстве и ремонте! Советы, обзоры материалов, технологии, тренды и новости отрасли. Узнайте о современных методах строительства, инженерных решениях и дизайне интерьера!
MarkNOlaf
| #
bitcoin dark web https://kingdomdarkmarketplace.com – best darknet markets
Volodyanaf
| #
darknet markets links https://darkode-market.com/ – dark websites
Donaldlaf
| #
darkmarket link darkmarket list
FNDaviditabe
| #
dark web marketplaces https://firstdarkmarket.com/ – darkmarket link
Toliknaf
| #
dark web marketplaces tor drug market
WilliamIRrutty
| #
darkmarkets https://darknetmarketsonion.shop/ – bitcoin dark web
Pingrutty
| #
dark web market list darkmarket
Rabyitabe
| #
dark market link darknet markets links
Rickie Litteral
| #
https://lioleo.edu.vn/creatorcourse02/4.png.php?id=win-win-bet
Kxyufaita
| #
darknet markets onion address https://cyberdarkmarkets.com/ – darknet market list
VortexV5
| #
Подкаст о https://schule-am-volkspark.de/plusminus-umfrage бизнесе.
Michaelimami
| #
frumzi.gr.com
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://rentalsea.ru/
DanielRak
| #
Аркада казино, или казино аркада, – это относительно новое направление в индустрии азартных игр, которое стремится сочетать элементы классических аркадных игр с традиционными казино-играми. Этот гибридный формат нацелен на привлечение более молодой аудитории, выросшей на видеоиграх, и тех, кто ищет более интерактивный и захватывающий опыт, нежели стандартные слоты и настольные игры. аркада казино бонусы
1win_seml
| #
1win. com http://1win112.com.kg/ .
MarkNOlaf
| #
dark market url https://cannahomedarknetdrugstore.com/ – dark web markets
DonDonfaita
| #
darknet markets 2025 darknet market links
1win_ajkn
| #
1вин сайт https://www.1win113.com.kg .
Volodyanaf
| #
dark web market list https://darkmarketpremium.com/ – darknet websites
Jamesenved
| #
Автополив участка – это идеальное решение для тех, кто ценит время и хочет эффективно ухаживать за своим садом и газоном. Наша ландшафтная студия предлагает полный спектр услуг по проектированию, установке и обслуживанию автоматических систем орошения. С помощью современного оборудования вы сможете легко и быстро полить ваш дачный участок, теплицу или парк. Наши специалисты проведут пуско-наладочные работы, настроят систему под ваши нужды и обеспечат качественное обслуживание. Не упустите возможность упростить уход за вашим озеленением с помощью автополива: сайт
1win_qypi
| #
1 win https://1win713.ru .
Donaldlaf
| #
darknet site darkmarket
Pingrutty
| #
dark market link dark web market urls
FNDaviditabe
| #
darknet market https://onion-dark-market.shop/ – dark web marketplaces
Toliknaf
| #
darknet drug links darknet drug market
WilliamIRrutty
| #
darknet marketplace https://kingdomdarkmarketonline.com/ – onion dark website
Rabyitabe
| #
darkmarkets dark market link
Michaelsoifs
| #
Glory Casino
Kxyufaita
| #
dark market 2025 https://cyberdarkmarkets.com/ – dark market link
Gianna Hardridge
| #
https://lioleo.edu.vn/christmas-section/4.png.php?id=8364-bet
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://ramregion.ru/
Michaelsoifs
| #
Glory Casino
MarkNOlaf
| #
dark markets https://cannahomedarknetdrugstore.com/ – darkmarket link
Volodyanaf
| #
darknet markets 2025 https://darkfoxdarkweb.com/ – tor drug market
DonDonfaita
| #
darknet drugs dark web drug marketplace
Pingrutty
| #
darkmarkets dark web market urls
1win_njml
| #
1 вин вход в личный кабинет http://1win112.com.kg/ .
1win_uakn
| #
1win официальный сайт вход 1win113.com.kg .
FNDaviditabe
| #
dark web link https://firstdarkmarket.com/ – dark web market links
Donaldlaf
| #
dark web market urls darknet markets
WilliamIRrutty
| #
dark web market https://kingdomdarkmarketonline.com/ – darkmarkets
Terencesam
| #
спины за регистрацию без депозита с выводом Играть игровые автоматы на деньги
Toliknaf
| #
darknet drug market dark web market links
1win_ippi
| #
1win зайти 1win зайти .
Michaelsoifs
| #
Glory Casino
Kxyufaita
| #
darknet markets onion address https://cannahome-darkmarket-online.com/ – darkmarket 2025
Rabyitabe
| #
darknet market darknet marketplace
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://imcongroup.ru/
Kartu Poker
| #
thanks everything will change after you post this blog
MarkNOlaf
| #
dark market 2025 https://kingdom-darkmarketplace.com – darkmarket link
AlphaV1
| #
Как не сдаться при изучении бизнеса: https://aroagardenbar.com.br/hello-world-2/
Molly Arbogust
| #
https://lioleo.edu.vn/christmas-section/4.png.php?id=win-bet-88
Pingrutty
| #
dark web markets darknet markets links
DonDonfaita
| #
darknet sites dark markets
Michaelsoifs
| #
Glory Casino
mtsekaterinburgsig
| #
мтс екатеринбург
https://timviec24h.com.vn/companies/24inetrnet-domashnij-ekb-3/
мтс тв
Michaelsoifs
| #
Glory Casino
FNDaviditabe
| #
tor drug market https://darkmarketdarkfox.com/ – darkmarket
Michaelimami
| #
https://frumzi.gr.com/
Michaelsoifs
| #
Glory Casino
Michaelsoifs
| #
Glory Casino
Donaldlaf
| #
dark web link darknet markets url
WilliamIRrutty
| #
dark web market https://kingdommarketdarknet.com/ – darknet market list
Michaelsoifs
| #
Glory Casino
Toliknaf
| #
darknet marketplace dark web market links
Kxyufaita
| #
darknet markets https://kingdomdarknetdrugstore.com/ – dark markets
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://realsfera.ru/
Rabyitabe
| #
darknet markets onion address tor drug market
Michaelsoifs
| #
Glory Casino
Pingrutty
| #
darknet market links dark market list
Volodyanaf
| #
dark markets 2025 https://darkwebversus.com/ – darknet market list
DonDonfaita
| #
tor drug market dark markets
FNDaviditabe
| #
darknet markets https://kingdom-marketplace.com/ – darknet markets 2025
webpage
| #
Incredible points. Outstanding arguments. Keep up the amazing
effort.
WilliamIRrutty
| #
tor drug market https://darknetmarketsonion.shop/ – dark web market
Donaldlaf
| #
dark market onion dark market
prodvijenie saitov v moskve_qlpt
| #
сео сайта prodvizhenie-sajtov-v-moskve221.ru .
prodvijenie saitov v moskve_nvKl
| #
заказать продвижение сайтов заказать продвижение сайтов .
WilliamDog
| #
Всё для мужчин https://kompanion.com.ua в одном месте! Спорт, мода, авто, гаджеты, здоровье и лайфхаки. Читай полезные советы, следи за трендами и будь лучшей версией себя.
HenryTut
| #
Новости шоу-бизнеса https://mediateam.com.ua мода, красота и афиша – всё в одном месте! Узнавайте о главных событиях, свежих трендах и топовых мероприятиях. Будьте в курсе всего, что происходит в мире стиля и развлечений!
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://talisman-65.ru/
Kxyufaita
| #
dark markets https://cannahome-darkmarket-online.com/ – tor drug market
Aaronaleft
| #
Все автомобильные новости https://nmiu.org.ua в одном месте! Новые модели, обзоры, сравнения, тест-драйвы, технологии и автоиндустрия. Следите за тенденциями и оставайтесь в курсе событий автопрома!
Pingrutty
| #
dark market list dark market link
Rabyitabe
| #
onion dark website darknet drugs
MarkNOlaf
| #
darknet links https://dark-web-versus.com – tor drug market
mtsekaterinburgsig
| #
мтс домашний интернет екатеринбург
https://stepstage.fr/employer/24inetrnet-domashnij-ekb/
мтс подключить
GeorgeTom
| #
Source:
https://eurekatomsk.ru/
Sazrmtn
| #
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по выгодным тарифам. Цена зависит от определенной специальности, года получения и образовательного учреждения. Стараемся поддерживать для клиентов адекватную политику цен. Важно, чтобы дипломы были доступны для большого количества граждан. купить диплом форум
Blue_Reaper
| #
Что лучше выбрать в бизнесе? https://kubalitours.com/family-friendly-resorts-in-coastal-kenya/
Cazrjlx
| #
Привет!
Мы изготавливаем дипломы любой профессии по приятным тарифам. Стоимость будет зависеть от определенной специальности, года получения и университета: rdiploman.com/
Uazrpsw
| #
Здравствуйте!
Для некоторых людей, заказать диплом о высшем образовании – это необходимость, возможность получить отличную работу. Но для кого-то – это желание не терять огромное количество времени на учебу в университете. С какой бы целью вам это не потребовалось, мы готовы помочь. Быстро, профессионально и выгодно сделаем диплом нового или старого образца на подлинных бланках со всеми печатями.
Главная причина, почему многие люди прибегают к покупке документов, – получить определенную работу. Например, навыки и опыт дают возможность человеку устроиться на работу, однако документального подтверждения квалификации нет. Если работодателю важно наличие “корочек”, риск потерять вакантное место очень высокий.
Приобрести документ ВУЗа можно в нашей компании в Москве. Мы предлагаем документы об окончании любых ВУЗов РФ. Вы сможете получить диплом по любой специальности, любого года выпуска, в том числе документы старого образца. Даем 100% гарантию, что в случае проверки документа работодателем, подозрений не возникнет.
Ситуаций, которые вынуждают заказать диплом ВУЗа очень много. Кому-то очень срочно требуется работа, в итоге необходимо произвести особое впечатление на начальство на протяжении собеседования. Некоторые хотят устроиться в престижную компанию, чтобы повысить собственный статус в обществе и в последующем начать собственное дело. Чтобы не тратить драгоценное время, а сразу начать удачную карьеру, используя имеющиеся знания, можно приобрести диплом в интернете. Вы сможете быть полезным в социуме, обретете финансовую стабильность максимально быстро и просто- купить диплом техникума
DonDonfaita
| #
darknet markets onion address darknet marketplace
FNDaviditabe
| #
dark web market list https://kingdom-marketplace.com/ – dark web market urls
WilliamIRrutty
| #
dark market link https://asap-market-onion.com/ – dark market 2025
Donaldlaf
| #
darknet websites darknet markets onion
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://brigantina-stroy.ru/
Pingrutty
| #
dark web market urls darknet markets onion
Kxyufaita
| #
dark web market https://cannahomemarket.link/ – darknet drug market
Toliknaf
| #
darkmarket url darknet site
Franksaumn
| #
Актуальные новости бизнеса https://rentwell.in.ua экономики и недвижимости! Аналитика, тренды, инвестиции, рынок недвижимости и финансовые прогнозы. Будьте в курсе главных событий и принимайте взвешенные решения!
JamesLep
| #
Выбор недвижимости в Киеве https://odinden.com.ua на что обратить внимание? Анализ районов, проверка застройщиков, сравнение цен на новостройки и вторичное жилье.
Franciswealk
| #
Лучший женский портал https://rosetti.com.ua Стиль, красота, здоровье, семья, карьера и саморазвитие. Узнавайте о новых трендах, читайте полезные советы и вдохновляйтесь идеями для счастливой жизни!
MarkNOlaf
| #
dark market url https://drdarkfoxmarket.com – darkmarket link
Volodyanaf
| #
dark web drug marketplace https://darkwebversus.com/ – best darknet markets
Rabyitabe
| #
darkmarket link darkmarket link
Terencesam
| #
bezdep Играть игровые автоматы на деньги
Odellnus
| #
https://cross.expert/partner/mrt-pozvonochnika-vazhnyy-metod-diagnostiki.html
DonDonfaita
| #
dark web link dark web market urls
FNDaviditabe
| #
darknet markets https://kingdom-marketplace.com/ – dark websites
lkjhCype
| #
http://dentaone.ru/wp-content/pages/uzghasu_na_kinogo___parad_igolok_dlya_nervov_i_fantaziy.html кинопремьеры смотреть онлайн бесплатно в хорошем качестве
WilliamIRrutty
| #
darknet drug market https://darknetmarketsonion.shop/ – darkmarket list
Pingrutty
| #
dark market list dark web market urls
mostbet_yemt
| #
mostbet игры https://mostbet1011.com.kg/ .
mostbet_ufMr
| #
мрстбет http://www.mostbet1004.com.kg .
Markcen
| #
Хотите сдать квартиру в аренду? Читайте проверенные советы https://kazhdogopravo.ru/
Kxyufaita
| #
dark market list https://cyberdarkmarkets.com/ – dark market 2025
Donaldlaf
| #
dark web marketplaces tor drug market
1win_ng_ifSi
| #
1 win nigeria 1 win nigeria .
mtsekaterinburgsig
| #
мтс тарифы на интернет
https://www.workinternational-df.com/employer/24inetrnet-domashnij-ekb-3/
сайт мтс екатеринбург
Michaelsoifs
| #
Glory Casino
Toliknaf
| #
darknet markets links onion dark website
Stephenloole
| #
Современный женский портал https://womanlife.kyiv.ua мода, стиль, красота, здоровье, отношения и карьера. Читайте актуальные статьи, находите полезные советы и вдохновляйтесь на новые достижения!
Volodyanaf
| #
darknet site https://darkmarketpremium.com/ – dark websites
MarkNOlaf
| #
darknet site https://kingdom-darkmarketplace.com – darknet sites
HaroldKnita
| #
Все для женщин https://timelady.kyiv.ua в одном месте! Новости моды, бьюти-советы, отношения, карьера, психология и лайфхаки. Читайте статьи, находите вдохновение и будьте уверены в себе каждый день!
Jordanmip
| #
Детский портал о здоровье https://run.org.ua полезная информация для заботливых родителей! Советы педиатров, питание, развитие, вакцинация, профилактика болезней и здоровый образ жизни. Всё, что нужно знать о здоровье вашего ребенка!
Michaelsoifs
| #
Glory Casino
Rabyitabe
| #
dark websites bitcoin dark web
Peternig
| #
The cryptocurrency market remains a whirlwind of activity, with Bitcoin price predictions oscillating wildly amidst global economic uncertainty. Ethereum’s market analysis reveals a struggle to maintain momentum, weighed down by network congestion and scaling challenges, even as the Merge’s long-term potential remains undeniable. Investors are keenly eyeing top altcoins, searching for the next breakout star, while bracing themselves for potential market corrections. Blockchain adoption updates
Charlescon
| #
накрутка дешево
Pingrutty
| #
darknet drug market darkmarket url
FNDaviditabe
| #
darknet markets onion https://darkmarketdarkfox.com/ – darkmarket
DonDonfaita
| #
best darknet markets dark market url
WilliamIRrutty
| #
dark web sites https://darknetmarketsonion.shop/ – darknet markets 2025
frumzi_Wob
| #
https://frumzi.xn--qxam/
RedV3
| #
Повышение эффективности через бизнес: https://asesinosasueldo.org/busco-un-sicario/.
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://1domovoy.ru/
Hubertfoend
| #
https://salda.ws/f/topic.php?f=12&t=81373
Kxyufaita
| #
darkmarket https://darkfoxmarketplace.com/ – darknet market list
lkjhCype
| #
https://rumol.ru/wp-includes/wli/smotret_serialu_onlayn___udobstvo_i_vubor_dlya_kazghdogo.html фильмы для просмотра на вечер
Donaldlaf
| #
darknet markets 2025 dark web link
sohbet
| #
great blog very nice articles I will follow you continuously sohbet odaları
Volodyanaf
| #
darknet market links https://darkmarketpremium.com/ – onion dark website
mostbet_ygmt
| #
mostbet kg скачать на андроид mostbet kg скачать на андроид .
MarkNOlaf
| #
darknet market list https://kingdom-darkmarketplace.com – darknet drugs
mostbet_omMr
| #
mostbet скачать https://mostbet1004.com.kg/ .
localtorontomovers
| #
toronto moving and storage company movers toronto
LandonOveta
| #
Всё для женщин https://allwoman.kyiv.ua в одном месте! Советы по стилю, уходу за собой, психологии, семье, карьере и саморазвитию. Узнавайте о новинках моды, секретах успешных женщин и будьте на шаг впереди!
Jimmyjab
| #
Современный женский сайт https://dama.kyiv.ua красота, стиль, здоровье, семья и карьера. Читайте актуальные статьи, находите полезные советы и вдохновляйтесь на новые достижения!
Rabyitabe
| #
dark market list best darknet markets
1win_ng_ntSi
| #
1win online games https://1win11.com.ng/ .
Pingrutty
| #
darknet sites dark web marketplaces
binance註冊獎金
| #
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
FNDaviditabe
| #
darknet market lists https://kingdom-marketplace.com/ – dark market list
mtsekaterinburgsig
| #
мтс подключить
http://modiyil.com/profile/will2470972029
мтс подключение
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://anrespectplus.ru/
WilliamIRrutty
| #
darknet drugs https://kingdomdarkmarketonline.com/ – best darknet markets
DonDonfaita
| #
dark market 2025 onion dark website
Kxyufaita
| #
darkmarket 2025 https://cannahome-darkmarket-online.com/ – darknet drug market
Volodyanaf
| #
darknet drugs https://darkode-market.com/ – darknet sites
Donaldlaf
| #
onion dark website darknet marketplace
MarkNOlaf
| #
dark market onion https://drdarkfoxmarket.com – darknet marketplace
Toliknaf
| #
darkmarkets darknet markets 2025
Pingrutty
| #
dark web market dark web link
Rabyitabe
| #
dark web market best darknet markets
FNDaviditabe
| #
darknet markets 2025 https://onion-dark-market.shop/ – darkmarkets
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://17metrov.ru/
WilliamIRrutty
| #
darkmarket 2025 https://asap-market-onion.com/ – darknet markets 2025
DonDonfaita
| #
tor drug market dark web link
Michaelles
| #
Женский онлайн-журнал https://krasotka.kyiv.ua мода, красота, здоровье, семья и карьера. Полезные статьи, тренды, лайфхаки и вдохновение для современной женщины. Читайте, развивайтесь и наслаждайтесь жизнью!
Moshebuh
| #
Онлайн-журнал для женщин https://otnoshenia.net которые стремятся к лучшему! Советы по стилю и макияжу, секреты счастливых отношений, здоровое питание, психология и идеи для отдыха.
Danieljoymn
| #
Лучший сайт для женщин https://model.kyiv.ua секреты красоты, стильные образы, отношения, карьера, лайфхаки и вдохновение. Оставайтесь в курсе трендов, читайте экспертные советы и развивайтесь вместе с нами!
Kxyufaita
| #
dark web market https://darkfoxmarketplace.com/ – darknet drugs
lkjhCype
| #
https://rosohrancult.ru/wp-includes/pages/filmu_142.html пастбище на лесной поляне 5 букв сканворд
Volodyanaf
| #
dark web sites https://darkode-market.com/ – darknet markets onion
DragonV3
| #
Что нельзя делать с бизнесом? https://seointellect.com/2023/04/09/boost-your-online-presence-our-top-digital-marketing/
ArthurElelp
| #
https://wiki.lovettcreations.org/index.php/Voditel_71V
MarkNOlaf
| #
darknet site https://cannahomedarknetdrugstore.com/ – dark web market list
Pingrutty
| #
dark market darkmarket list
ArthurElelp
| #
https://dptotti.fic.edu.uy/mediawiki/index.php/Voditel_52o
Toliknaf
| #
darknet market list darknet drugs
Rabyitabe
| #
darknet sites dark web market urls
mtsekaterinburgsig
| #
мтс подключение екатеринбург
https://skillsdiscover.com/profile/anitaalcock302
мтс тарифы на интернет
FrankFiP
| #
https://akvilon152.ru
ArthurElelp
| #
http://kousokuwiki.org/wiki/%E5%88%A9%E7%94%A8%E8%80%85:CortezSpyer5372
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://ubkrent.ru/
GeorgeTom
| #
Source:
https://eurekatomsk.ru/
FNDaviditabe
| #
darkmarket list https://mydarkmarketlink.com/ – dark market link
Sazrlye
| #
Мы изготавливаем дипломы любой профессии по приятным тарифам. Преимущества покупки документов в нашем сервисе
Вы заказываете диплом через надежную фирму. Такое решение позволит вам сохранить не только массу средств, но и ваше драгоценное время.
Плюсов намного больше:
• Дипломы изготавливаются на подлинных бланках с мокрыми печатями и подписями;
• Дипломы любого ВУЗа России;
• Цена в разы ниже той, которую пришлось бы платить на очном обучении в университете;
• Доставка как по Москве, так и в другие регионы Российской Федерации.
Заказать диплом института– http://powerofmony.copiny.com/question/details/id/1061041/ – powerofmony.copiny.com/question/details/id/1061041
ArthurElelp
| #
https://techuswiki.xyz/index.php/Voditel_39A
WilliamIRrutty
| #
darknet market links https://idarknetmarket.com/ – dark market link
DonDonfaita
| #
dark web sites best darknet markets
Kxyufaita
| #
darknet sites https://darkfoxmarketplace.com/ – darknet websites
Volodyanaf
| #
dark web market links https://darkmarketpremium.com/ – darknet market list
Pingrutty
| #
darknet drug store darkmarket link
RalphPauth
| #
Сайт для современных женщин https://princess.kyiv.ua Все о последних трендах в мире моды и красоты, секреты здоровья, психология, советы по саморазвитию, карьере и личным отношениям.
JamesTef
| #
Портал для женщин https://woman365.kyiv.ua которые ценят красоту и уверенность! Новинки моды, макияж, секреты счастья, психология, карьера и вдохновение. Узнавайте полезные советы и воплощайте мечты в реальность!
GlennRon
| #
Женский онлайн-клуб https://womanclub.kyiv.ua для тех, кто ценит стиль, здоровье и успех! Узнайте секреты красоты, тренды моды, советы по отношениям и карьере. Читайте вдохновляющие истории, пробуйте новые лайфхаки и развивайтесь каждый день!
MarkNOlaf
| #
dark web drug marketplace https://cannahomedarknetdrugstore.com/ – onion dark website
Donaldlaf
| #
dark web market darknet markets
Toliknaf
| #
dark market dark websites
lkjhCype
| #
https://flashparade.ru/wp-includes/jks/novogodnie_filmu_onlayn_smotret_besplatno_v_horoshem_kachestve.html авито купить лук на перо
Rabyitabe
| #
darknet sites darknet markets url
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://kvadrolampa.ru/
FNDaviditabe
| #
darknet market links https://mydarkmarketlink.com/ – dark web market links
WilliamIRrutty
| #
darknet links https://asap-market-onion.com/ – dark market onion
Pingrutty
| #
darknet markets darkmarkets
Kxyufaita
| #
darknet market lists https://kingdomdarknetdrugstore.com/ – darknet markets links
Volodyanaf
| #
darknet sites https://darkfoxdarkweb.com/ – dark web link
DonDonfaita
| #
dark web market urls dark market url
Sazrjja
| #
Заказать документ ВУЗа можно у нас в Москве. Мы предлагаем документы об окончании любых университетов РФ. Вы получите диплом по любым специальностям, включая документы старого образца. Гарантируем, что в случае проверки документов работодателями, подозрений не появится. babygirls022.copiny.com/question/details/id/1069103
vpesports
| #
CS:GO/CS2 skins wiki.vpesports com catalog with full description! Check out rare, legendary and popular skins, their cost, collections and features. Find the perfect skin for your inventory!
MarkNOlaf
| #
dark market onion https://kingdomdarkmarketplace.com – darknet market links
AllenDow
| #
The best online marketplace https://warmbloodsales.com.au/social-media-account-marketplace/! Buy and sell game, social, business and other accounts. Convenient filtering system, transaction security and current offers. All popular services and games in one place!
Vodka slots
| #
Hello There. I discovered your weblog the usage of msn. This is an extremely neatly written article.
I’ll be sure to bookmark it and come back to learn extra of your useful
info. Thanks for the post. I’ll definitely comeback.
BertramSpode
| #
Buy verified accounts account market with ease! Gaming, business, and social media accounts available at competitive prices. Fast transactions, trusted sellers, and secure payments. Get the best deals now!
Donaldlaf
| #
darknet markets onion address dark web market urls
Night_Pioneer
| #
Видел прогнозы, что Бизнес будет главным https://www.aquilamanagement.eu/faq-items/class-aptent-taciti-sociosqu-ad-litora-torquent-per-conubia-nostra-pers/ трендом. Возражения?
Toliknaf
| #
dark market bitcoin dark web
1win_abot
| #
1 вин официальный http://www.1win715.ru .
1win_atkr
| #
1 win казино 1 win казино .
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://vam42.ru/
frumzi_Wob
| #
https://frumzi.xn--qxam/
1win_lgPr
| #
1win.am http://www.1win708.ru .
FNDaviditabe
| #
dark market link https://onion-dark-market.shop/ – darkmarket
Iariorxta
| #
Заказать документ института вы можете у нас. netione.consulting/blog/image-minus-id-quod
BarrySoB
| #
Модульные дома — это идеальное решение для тех, кто ценит комфорт и стиль. Современные одноэтажные и двухэтажные конструкции позволяют создать уютное загородное жильё. Мы предлагаем домокомплекты, которые включают всё необходимое: от проектирования до отделки. Удобная планировка обеспечит комфорт в каждой комнате, а качественное утепление и отопление сделают дом тёплым и уютным. Постройка проходит быстро и по доступной цене. Создайте свой идеальный дом с нашей помощью – https://pkf-stroitel.ru/
Mazrifh
| #
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по приятным тарифам. Стараемся поддерживать для клиентов адекватную ценовую политику. Для нас важно, чтобы документы были доступны для большого количества граждан.
Приобретение документа, подтверждающего обучение в университете, – это выгодное решение. Приобрести диплом о высшем образовании: diplomist.com/kupit-diplom-kolledzha-33/
Jariorkgj
| #
Где купить диплом по необходимой специальности?
Мы предлагаем выгодно приобрести диплом, который выполнен на оригинальном бланке и заверен мокрыми печатями, штампами, подписями должностных лиц. Документ способен пройти любые проверки, даже при использовании специального оборудования. Достигайте цели быстро и просто с нашей компанией.
Приобрести диплом ВУЗа diplomc-v-ufe.ru/kupit-diplom-yurista-12/
WilliamIRrutty
| #
darkmarket list https://kingdommarketdarknet.com/ – dark market 2025
Pingrutty
| #
dark websites dark market list
Volodyanaf
| #
darknet links https://aasap-market.com/ – darkmarket link
Kxyufaita
| #
darknet markets 2025 https://darkfoxmarketplace.com/ – dark market url
DonDonfaita
| #
dark web drug marketplace https://github.com/darkwebwebsites/darkwebwebsites onion dark website
Terencesam
| #
Бесплатные спины за регистрацию без депозита Бонус без отыгрыша
MarkNOlaf
| #
dark market 2025 https://cannahomedarknetdrugstore.com/ – darknet market lists
Rabyitabe
| #
darknet market list https://github.com/darknetmarketlinks2025/darknetmarkets dark markets
DavidEthef
| #
Единственный знак зодиака, который имеет особую связь с Богом https://x.com/Fariz418740/status/1901024008243429625
Donaldlaf
| #
dark market https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets 2025
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://uralwood45.ru/
Toliknaf
| #
darknet market links https://github.com/darknetmarketslist/darknetmarketslist dark web market urls
Pingrutty
| #
dark markets 2025 https://github.com/darknetmarkets2025/darknetmarketlinks darknet market list
FNDaviditabe
| #
darknet markets onion https://darkmarketdarkfox.com/ – dark web market urls
WilliamIRrutty
| #
darknet drug store https://idarknetmarket.com/ – darkmarket
1win_hdot
| #
1win личный кабинет https://1win715.ru .
1win_iqkr
| #
1win партнерка вход https://www.1win114.com.kg .
Volodyanaf
| #
darkmarket link https://aasap-market.com/ – darknet drug links
Kxyufaita
| #
dark web marketplaces https://cannahomemarket.link/ – bitcoin dark web
1win_tqPr
| #
1 win.am https://www.1win708.ru .
DonDonfaita
| #
onion dark website https://github.com/darkwebwebsites/darkwebwebsites onion dark website
MarkNOlaf
| #
darknet markets url https://drdarkfoxmarket.com – darknet drugs
Kennethenubs
| #
Единственный знак зодиака, который имеет особую связь с Богом https://www.pinterest.com/pin/957366833290360277
BarrySoB
| #
Модульные дома – это современное и удобное жильё, которое можно быстро построить. Они имеют гибкую планировку, что позволяет создать стильный и комфортный интерьер. У нас вы найдете домокомплекты для одноэтажных и двухэтажных домов, а также загородных коттеджей. Строительство модульного дома включает в себя качественные материалы, утепление и удобную сантехнику. Вы можете выбрать отделку, мебель и дизайн по вашему вкусу. Постройте свой идеальный дом с максимальным комфортом для семьи https://pkf-stroitel.ru/
Rabyitabe
| #
darknet market https://github.com/darknetmarketlinks2025/darknetmarkets dark web market links
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://geogroupeng.ru/
Donaldlaf
| #
dark web market https://github.com/darkwebmarketslinks/darkwebmarkets darkmarket url
Pingrutty
| #
darknet drug store https://github.com/darknetmarkets2025/darknetmarketlinks dark markets
Master3373
| #
Давайте обсудим о бизнесе: https://regonequipments.com/2020/01/06/hello-world/.
Toliknaf
| #
dark market list https://github.com/darknetmarketslist/darknetmarketslist dark market onion
FNDaviditabe
| #
darknet drug market https://kingdom-marketplace.com/ – dark web market list
WilliamIRrutty
| #
darknet websites https://kingdommarketdarknet.com/ – darknet markets onion address
Volodyanaf
| #
dark web market urls https://darkode-market.com/ – darknet drug store
Kxyufaita
| #
darknet markets links https://cannahome-darkmarket-online.com/ – darkmarket link
DonDonfaita
| #
darkmarkets https://github.com/darkwebwebsites/darkwebwebsites darknet drug store
MarkNOlaf
| #
darknet markets 2025 https://cannahomedarknetdrugstore.com/ – darknet markets onion address
Pingrutty
| #
darkmarket link https://github.com/darknetmarkets2025/darknetmarketlinks dark web market links
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://stroyk-wood.ru/
Rabyitabe
| #
darknet site https://github.com/darknetmarketlinks2025/darknetmarkets darknet markets 2025
CharlesNew
| #
Для складских помещений оптимальный выбор – полимерные полы от этого производителя https://www.bandlab.com/miron91
Donaldlaf
| #
dark web marketplaces https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets
JamesNeumn
| #
Лучший автомобильный портал https://kia-sportage.in.ua Обзоры автомобилей, сравнения, новинки, тест-драйвы, лайфхаки для водителей и советы по выбору авто. Всё, что нужно автолюбителям и профессионалам!
RonaldIdori
| #
Сайт для женщин https://womanexpert.kyiv.ua которые хотят быть успешными и счастливыми! Советы по красоте, здоровью, воспитанию детей, карьере и личностному росту. Актуальные тренды, лайфхаки и вдохновение для современных женщин!
Julianzed
| #
Портал для автолюбителей https://lada.kharkiv.ua всё об автомобилях! Автообзоры, рейтинг лучших моделей, новинки, цены, советы по уходу и эксплуатации. Узнайте, какие авто заслуживают вашего внимания!
FNDaviditabe
| #
darkmarket https://darkmarketdarkfox.com/ – dark web market links
Toliknaf
| #
dark web market links https://github.com/darknetmarketslist/darknetmarketslist darknet drug links
WilliamIRrutty
| #
darknet market lists https://idarknetmarket.com/ – darknet market
CharlesNew
| #
Компания разработала серию эпоксидных смол для создания изделий в технике “геод”, все необходимые материалы и мастер-классы по ссылке https://conifer.rhizome.org/Anton998
Volodyanaf
| #
darknet drug links https://darkwebversus.com/ – darknet marketplace
GeorgeTom
| #
Source:
https://eurekatomsk.ru/
Kxyufaita
| #
darknet drug store https://cyberdarkmarkets.com/ – dark web marketplaces
MarkNOlaf
| #
darkmarkets https://cannahomedarknetdrugstore.com/ – dark web link
lkjhCype
| #
https://legonew.ru/files/price/multfilmu_pro_kosmos___zahvatuvaushiy_mir_dlya_detey_i_vzrosluh.html американские фильмы 2000 2010 годов
Pingrutty
| #
darknet market https://github.com/darknetmarkets2025/darknetmarketlinks darkmarket list
DonDonfaita
| #
dark market 2025 https://github.com/darkwebwebsites/darkwebwebsites dark web market list
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://cemstroy-t68.ru/
Sazryce
| #
Заказ официального диплома через надежную фирму дарит ряд плюсов для покупателя. Это решение дает возможность сэкономить время и значительные финансовые средства. Впрочем, преимуществ гораздо больше.Мы можем предложить дипломы любой профессии. Дипломы производят на оригинальных бланках. Доступная стоимость по сравнению с крупными расходами на обучение и проживание. Покупка диплома о высшем образовании из российского университета является разумным шагом.
Приобрести диплом о высшем образовании: diplomc-v-ufe.ru/kupit-attestati-za-11-klass-bistro-i-udobno/
Rabyitabe
| #
darknet market lists https://github.com/darknetmarketlinks2025/darknetmarkets darkmarket 2025
Pioneer3892
| #
Лично мне помог этот ресурс по бизнесу: https://kelidsazan.com/%da%a9%d9%84%db%8c%d8%af%d8%b3%d8%a7%d8%b2-%d8%a7%d8%b4%d8%b1%d9%81%db%8c-%d8%a7%d8%b5%d9%81%d9%87%d8%a7%d9%86%db%8c/
Donaldlaf
| #
darknet market https://github.com/darkwebmarketslinks/darkwebmarkets dark web marketplaces
Terencesam
| #
Игровые автоматы на деньги лучшие Бонус с выводом без депозита
FNDaviditabe
| #
dark websites https://firstdarkmarket.com/ – dark web markets
WilliamIRrutty
| #
darknet marketplace https://darknetmarketsonion.shop/ – darknet markets onion address
Toliknaf
| #
darknet markets onion address https://github.com/darknetmarketslist/darknetmarketslist dark market 2025
Volodyanaf
| #
onion dark website https://aasap-market.com/ – darknet markets onion
pinco kazino _jxSn
| #
pinco kazino pinco kazino .
WilburnJasse
| #
Выявлен фрукт, который является природным эликсиром молодости https://www.pinterest.com/pin/957366833290370789
Kxyufaita
| #
darknet drugs https://cannahomemarket.link/ – darknet markets
profilnaya tryba_koKt
| #
профильная труба оптом proftruba-moscow.ru .
propysk v moskvy dlya gryzovikov_hyol
| #
пропуск в центр москвы для грузовиков цена пропуск в центр москвы для грузовиков цена .
Pingrutty
| #
dark web market urls https://github.com/darknetmarkets2025/darknetmarketlinks dark web market links
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://anapa-centrstroy.ru/
MarkNOlaf
| #
dark web drug marketplace https://cannahomedarknetdrugstore.com/ – darkmarkets
DonDonfaita
| #
tor drug market https://github.com/darkwebwebsites/darkwebwebsites darknet markets 2025
Rabyitabe
| #
dark web market urls https://github.com/darknetmarketlinks2025/darknetmarkets darkmarket url
FNDaviditabe
| #
darknet drug market https://firstdarkmarket.com/ – darknet site
Donaldlaf
| #
bitcoin dark web https://github.com/darkwebmarketslinks/darkwebmarkets darkmarket link
WilliamIRrutty
| #
dark markets 2025 https://kingdommarketdarknet.com/ – dark web market list
Volodyanaf
| #
darknet sites https://darkmarketpremium.com/ – darknet market lists
Toliknaf
| #
dark web drug marketplace https://github.com/darknetmarketslist/darknetmarketslist tor drug market
Thomascoilk
| #
Which Zodiac signs should be careful – a dangerous period begins tomorrow! https://x.com/Fariz418740/status/1901169237206397369
Pingrutty
| #
dark websites https://github.com/darknetmarkets2025/darknetmarketlinks darknet site
RobertVow
| #
казино онлайн Казино всегда манили азартом и возможностью быстро разбогатеть. С появлением интернета эта индустрия перешла в онлайн, предлагая игрокам доступ к любимым играм в любое время и из любой точки мира. Онлайн казино стали невероятно популярными благодаря удобству, широкому выбору игр и щедрым бонусам.
Kxyufaita
| #
darknet marketplace https://kingdomdarknetdrugstore.com/ – dark market
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://ceramodecol.ru/
lkjhCype
| #
http://www.newlcn.com/pages/news/filmu_141.html один книга про мальчика на острове
MarkNOlaf
| #
tor drug market https://cannahomedarknetdrugstore.com/ – bitcoin dark web
Kennethsib
| #
Сайт для женщин https://womanportal.kyiv.ua всё о моде, красоте, здоровье, отношениях и саморазвитии! Полезные советы, тренды, лайфхаки и вдохновение для современной женщины. Будьте стильной, уверенной и успешной!
JamesDap
| #
Автомобильный сайт https://newsgood.com.ua для водителей и автолюбителей! Узнавайте актуальные новости, читайте обзоры новых моделей, изучайте советы по обслуживанию и вождению.
Williamnof
| #
Авто портал для всех https://rupsbigbear.com кто любит автомобили! Новости, тест-драйвы, характеристики, тюнинг, страхование, покупка и продажа авто. Следите за последними тенденциями и выбирайте лучшее для себя!
DonDonfaita
| #
dark web market list https://github.com/darkwebwebsites/darkwebwebsites dark market url
FNDaviditabe
| #
darknet links https://mydarkmarketlink.com/ – darknet drug market
Rabyitabe
| #
darknet markets links https://github.com/darknetmarketlinks2025/darknetmarkets dark web markets
Blue_Nomad
| #
Инфографика по бизнесу: http://inetlog.ru/
Donaldlaf
| #
dark web drug marketplace https://github.com/darkwebmarketslinks/darkwebmarkets dark markets 2025
Volodyanaf
| #
darknet markets 2025 https://darkmarketpremium.com/ – best darknet markets
WilliamIRrutty
| #
tor drug market https://asap-market-onion.com/ – dark market link
Pingrutty
| #
dark web marketplaces https://github.com/darknetmarkets2025/darknetmarketlinks dark market
Toliknaf
| #
darknet marketplace https://github.com/darknetmarketslist/darknetmarketslist dark web market links
Kxyufaita
| #
darknet drug store https://cannahomemarket.link/ – onion dark website
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные рекомендации — https://domnabakalinskoy.ru/
MarkNOlaf
| #
dark web market links https://kingdom-darkmarketplace.com – darkmarket url
FrankFiP
| #
https://best-kuzminki.ru
DonDonfaita
| #
dark markets https://github.com/darkwebwebsites/darkwebwebsites dark market url
Poker
| #
Hi there very very impressive bloggg euy!! Guy.. Excellent.. Awesome.. I’ll bookmark your site euy and take the feeds additionally yes? may i? I am glad to find so many useful information right here euy within the submit, we need develop extra strategies on this regard, thank you euy for sharing……
Pingrutty
| #
darknet markets links https://github.com/darknetmarkets2025/darknetmarketlinks darknet market links
FNDaviditabe
| #
darkmarkets https://firstdarkmarket.com/ – darknet site
Volodyanaf
| #
darknet marketplace https://darkode-market.com/ – dark web market
Rabyitabe
| #
dark markets https://github.com/darknetmarketlinks2025/darknetmarkets darknet markets url
prodvijenie saitov v moskve_dlPa
| #
заказать seo заказать seo .
Donaldlaf
| #
darknet site https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets onion
Lucienboymn
| #
Which Zodiac signs should be careful – a dangerous period begins tomorrow!
https://www.pinterest.com/pin/957366833290379120
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://akvilon152.ru/
Kxyufaita
| #
dark markets 2025 https://cannahome-darkmarket-online.com/ – tor drug market
Toliknaf
| #
bitcoin dark web https://github.com/darknetmarketslist/darknetmarketslist darknet drugs
Gregoryfaf
| #
Курск проститутки
MarkNOlaf
| #
best darknet markets https://dark-web-versus.com – darknet websites
DonDonfaita
| #
darknet site https://github.com/darkwebwebsites/darkwebwebsites darknet drug store
Sazrazz
| #
купить диплом дерматолога
FNDaviditabe
| #
darknet markets url https://onion-dark-market.shop/ – dark web marketplaces
Trefptg
| #
Заказать документ ВУЗа вы можете в нашем сервисе. diplomskiy.com/kupit-diplom-elektrika-2
Volodyanaf
| #
dark market https://darkode-market.com/ – darknet markets links
binance registration
| #
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
WilliamIRrutty
| #
dark websites https://darknetmarketsonion.shop/ – bitcoin dark web
Rabyitabe
| #
dark web market https://github.com/darknetmarketlinks2025/darknetmarkets dark web drug marketplace
MichaelSon
| #
новости транспорта Казахстан – Skoda Octavia, последние новости Казахстан
Donaldlaf
| #
dark web sites https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets 2025
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://magnk.ru/
Hacker9918
| #
Что не пишут https://lnx.juliacom.it/2019/10/02/dietro-le-quinte/ в учебниках с бизнесом.
Kxyufaita
| #
darknet market lists https://kingdomdarknetdrugstore.com/ – dark web sites
Donalddus
| #
Kraken вход – Как зайти на кракен, Kraken зеркало
Toliknaf
| #
darkmarket list https://github.com/darknetmarketslist/darknetmarketslist dark web market
Charleswhano
| #
you can try this out https://1253658985244568521.com/
Harleyfurdy
| #
Монтаж проходит практически бесшумно и не доставляет неудобств соседям https://potolki-v-moskve.ru/
MarkNOlaf
| #
best darknet markets https://dark-web-versus.com – dark web drug marketplace
JewelExpek
| #
Usual Veda is at the forefront of blockchain development, providing innovative technology solutions for businesses and developers. Our expertise in decentralized systems and secure blockchain applications enables companies to optimize operations and stay ahead in the digital era. With Usual Veda, businesses gain access to reliable, scalable, and cutting-edge technology that drives success. https://usual-vault.com
Josephshica
| #
navigate to this web-site keplr wallet extension
Pingrutty
| #
darkmarket url https://github.com/darknetmarkets2025/darknetmarketlinks darknet market
PerryQuove
| #
Ethereal Trade is a cutting-edge decentralized exchange that redefines the way users trade cryptocurrencies. With a focus on security, speed, and efficiency, Ethereal Trade offers a seamless trading experience without intermediaries. As a leader in crypto trading, our platform ensures transparent transactions and advanced trading tools, making it the go-to solution for both beginners and experienced traders. https://ethereal.ac/
DonDonfaita
| #
darknet market list https://github.com/darkwebwebsites/darkwebwebsites darkmarket 2025
JamesKip
| #
Лучший женский онлайн-журнал https://sweetheart.kyiv.ua Всё о моде, красоте, здоровье, отношениях и успехе. Экспертные советы, лайфхаки и мотивация для уверенных в себе женщин.
CecilMairl
| #
Женский журнал онлайн https://sunshadow.com.ua стиль, уход, секреты красоты, семья, карьера и саморазвитие. Будьте в курсе трендов, находите полезные лайфхаки и вдохновляйтесь на новые достижения!
Erwinrap
| #
Ваш гид в мире красоты https://wonderwoman.kyiv.ua моды и саморазвития! Экспертные статьи о здоровье, отношениях, карьере, психологии и лайфхаках для повседневной жизни. Оставайтесь в курсе трендов и открывайте новые возможности!
mostbet_hgMl
| #
mostbet kg скачать https://mostbet1005.com.kg/ .
1win_idsn
| #
1win rossvya 1win rossvya .
FNDaviditabe
| #
dark web drug marketplace https://kingdom-marketplace.com/ – dark market
Volodyanaf
| #
darknet websites https://darkode-market.com/ – dark market
1win_mjKi
| #
1вин сайт онлайн http://www.1win709.ru .
WilliamIRrutty
| #
dark market list https://asap-market-onion.com/ – darknet markets 2025
mtsekaterinburgsig
| #
мтс домашний интернет
https://koutiem.com/profile/wilfredoheb07
мтс подключение
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://asksteel.ru/
Rabyitabe
| #
dark web marketplaces https://github.com/darknetmarketlinks2025/darknetmarkets darknet market links
Donaldlaf
| #
dark web markets https://github.com/darkwebmarketslinks/darkwebmarkets darkmarket
Kxyufaita
| #
dark web market https://cyberdarkmarkets.com/ – darkmarket 2025
LindsayWem
| #
криптовалюта Криптовалюта стремительно меняет ландшафт азартных игр. Анонимность, быстрые транзакции и отсутствие посредников привлекают как операторов, так и игроков. Биткоин, Эфириум и другие цифровые активы становятся все более популярным способом пополнения счетов и вывода выигрышей.
Toliknaf
| #
darknet websites https://github.com/darknetmarketslist/darknetmarketslist dark markets
Terencesam
| #
Игровые автоматы играть на деньги Игровые автоматы лучшие
Pingrutty
| #
dark market list https://github.com/darknetmarkets2025/darknetmarketlinks darkmarket url
MarkNOlaf
| #
darknet sites https://kingdom-darkmarketplace.com – dark web markets
lkjhCype
| #
http://vip-barnaul.ru/includes/pages/onlayn_filmu___komfort_i_dostupnost_v_mire_kinematografa.html старсинема нижневартовск афиша на сегодня
Volodyanaf
| #
darknet websites https://darkfoxdarkweb.com/ – best darknet markets
FNDaviditabe
| #
darkmarket list https://mydarkmarketlink.com/ – darknet drug links
WilliamIRrutty
| #
darkmarket 2025 https://kingdomdarkmarketonline.com/ – darknet drugs
RobertAnest
| #
Женский мир https://vsegladko.net без границ! Узнайте всё о моде, уходе за собой, фитнесе, психологии, карьере и хобби. Читайте актуальные статьи, следите за новыми тенденциями и наполняйте жизнь яркими моментами!
MichaelTRINO
| #
Автомобильный онлайн-журнал https://svobodomislie.com всё о мире авто! Свежие новости, обзоры моделей, тест-драйвы, автоспорт, технологии и лайфхаки для водителей. Следите за трендами и будьте в курсе последних событий автопрома!
JamesEscob
| #
Онлайн-журнал о машинах https://sw.org.ua всё, что нужно знать об автомобилях! Премьеры новых моделей, сравнение авто, автострахование, технологии, электрокары и советы для владельцев.
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://jilfondilim.ru/
Donaldlaf
| #
darknet markets url https://github.com/darkwebmarketslinks/darkwebmarkets darkmarkets
Pingrutty
| #
darknet site https://github.com/darknetmarkets2025/darknetmarketlinks dark web sites
Kxyufaita
| #
darknet links https://darkfoxmarketplace.com/ – darknet market links
mostbet_ukMl
| #
мотбет mostbet1005.com.kg .
1win_uysn
| #
игра ракета на деньги 1win http://1win714.ru/ .
Toliknaf
| #
best darknet markets https://github.com/darknetmarketslist/darknetmarketslist dark websites
MarkNOlaf
| #
darknet marketplace https://kingdomdarkmarketplace.com – onion dark website
1win_qyKi
| #
1winn https://www.1win709.ru .
AlphaZ6
| #
Лично мне помог этот ресурс по бизнесу: https://blog.12min.com/br/frases-marcantes-de-livros/
JesseTef
| #
Энвато Envato, Adobe Stock, iStock, Depositphotos, Dreamstime – это лишь верхушка айсберга в мире стоковых платформ, предлагающих фотографии, видео, графику и другие креативные ресурсы. Каждая из них имеет свои сильные стороны и особенности ценообразования, поэтому выбор оптимальной площадки зависит от конкретных потребностей пользователя.
Harleyfurdy
| #
При повреждении водой достаточно слить жидкость и восстановить натяжение https://potolki-v-moskve.ru/
DonDonfaita
| #
darknet markets 2025 https://github.com/darkwebwebsites/darkwebwebsites dark market 2025
Volodyanaf
| #
onion dark website https://aasap-market.com/ – bitcoin dark web
FNDaviditabe
| #
dark web markets https://darkmarketdarkfox.com/ – dark web drug marketplace
mtsekaterinburgsig
| #
сайт мтс екатеринбург
https://norsknflklubb.com/read-blog/6118_mts-ekaterinburg-podklyuchenie-k-seti-5g.html
мтс подключить
Pingrutty
| #
dark web market urls https://github.com/darknetmarkets2025/darknetmarketlinks dark market 2025
WilliamIRrutty
| #
darknet sites https://kingdomdarkmarketonline.com/ – dark websites
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://aurora-kazan.ru/
pinco kazino _wpmt
| #
pinco kazino az pinco kazino az .
Donaldlaf
| #
dark web market https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets
Roberthob
| #
Сайт о красоте и здоровье https://beautytips.kyiv.ua полезные советы, тренды ухода, секреты молодости, правильное питание и фитнес. Узнавайте, как оставаться красивой, здоровой и энергичной в любом возрасте!
ScottyGuice
| #
Современный женский https://adviceskin.com журнал для тех, кто хочет быть стильной, уверенной и успешной! Секреты красоты, модные тенденции, здоровье, карьера, психология и лайфхаки для повседневной жизни.
Josephtus
| #
Мода, красота и здоровье https://fashiontop.com.ua всё в одном месте! Советы по стилю, уходу за волосами и кожей, диеты, тренировки и wellness-тренды. Узнайте, как выглядеть и чувствовать себя на 100%!
Kxyufaita
| #
darknet drug links https://kingdomdarknetdrugstore.com/ – dark web markets
Toliknaf
| #
dark market https://github.com/darknetmarketslist/darknetmarketslist dark web market urls
MarkNOlaf
| #
dark web market list https://drdarkfoxmarket.com – darknet market links
lkjhCype
| #
http://neoko.ru/images/pages/filmu_onlayn__komfortnuy_sposob_naslazghdatsya_kino.html водии гургон дом кисми 1
Robertgrecy
| #
Когда состоится встреча Путина и Трампа? https://www.youtube.com/watch?v=QaQAFGjNvyM
PizaimoBap
| #
Срочно понадобились деньги, зашел в Яндекс и сразу нашел отличный вариант! Оформил все онлайн займы , одобрили быстро, условия отличные – всего 0.6% в день.
Sazrrzp
| #
Всегда считал, что покупка диплома о высшем образовании — это миф. Но, оказалось, что все возможно! Сначала искал информацию по теме: купить диплом пту ссср, куплю диплом высшего образования санкт петербург, можно купить диплом техникума, проведение диплома купить, купить диплом в кызыле, а затем перешел на дипломы вузов. Подробнее можно узнать здесь: kyc-diplom.com/geography/noginsk.html
DonDonfaita
| #
darkmarket url https://github.com/darkwebwebsites/darkwebwebsites darkmarket list
Pingrutty
| #
dark websites https://github.com/darknetmarkets2025/darknetmarketlinks darknet markets 2025
Lazrmbb
| #
Купить диплом университета!
Мы предлагаем дипломы любой профессии по приятным тарифам— good-diplom.ru/kupit-diplom-v-kurske-11/
Volodyanaf
| #
darkmarkets https://darkwebversus.com/ – dark web sites
FNDaviditabe
| #
darkmarket url https://darkmarketdarkfox.com/ – dark market onion
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://arenda-specstroy.ru/
WilliamIRrutty
| #
dark web link https://kingdomdarkmarketonline.com/ – darknet site
Donaldlaf
| #
dark web drug marketplace https://github.com/darkwebmarketslinks/darkwebmarkets dark market
JamesVerve
| #
Идеальный баланс стиля https://feromonia.com.ua красоты и здоровья! Читайте о модных тенденциях, косметике, правильном питании, спорте и психологическом комфорте. Найдите вдохновение для гармоничной жизни!
JerrySuelo
| #
Мода, красота и здоровье https://gryada.org.ua ваш путь к идеальному образу! Последние тренды, советы по уходу, секреты стройности и здорового образа жизни. Создавайте гармонию в своём стиле и самочувствии!
AngelKah
| #
Секреты идеального стиля https://ladyone.kyiv.ua молодости и здоровья! Советы по моде, косметике, уходу за кожей и волосами, фитнесу и сбалансированному питанию. Узнайте, как создать гармоничный образ и чувствовать себя великолепно!
Kxyufaita
| #
dark web market links https://cyberdarkmarkets.com/ – darknet markets onion
Toliknaf
| #
dark web marketplaces https://github.com/darknetmarketslist/darknetmarketslist darkmarket
"https://covid-wiki.info/index.php?title=Exsrocket.ru_32Z"
| #
Продал bitcoin через сервис, курс был очень выгодный.
“https://menwiki.men/wiki/Exsrocket.ru_49B”
MarkNOlaf
| #
onion dark website https://kingdom-darkmarketplace.com – darknet markets onion address
Pingrutty
| #
darknet markets links https://github.com/darknetmarkets2025/darknetmarketlinks dark web market urls
saratogaautobodymailm
| #
Discover the expertise of auto tuning at Saratoga Auto Body, where your vehicle’s performance is fully enhanced . Our skilled team offers bespoke tuning options designed to transform your car’s appearance and efficiency . Join our satisfied clientele and drive a vehicle that truly reflects your unique taste. Visit https://saratogaautobody.com/ and commence your car’s reimagining today!
JohnnyGibra
| #
Ищете надежную компанию для ремонта квартиры? Мы предлагаем полный спектр услуг: от капитального ремонта до отделки интерьера. Ваше жильё преобразится, а мы сделаем всё качественно и в срок. Занимаемся демонтажом, перепланировкой, заменой сантехники и укладкой плитки. Мы работаем с разными проектами — однокомнатный, двух-, трех- и четырехкомнатный квартиры. Гарантия на все работы! Звоните, чтобы узнать стоимость и получить профессиональную помощь. Ваш идеальный дом уже ждет вас: отделка квартир
Michaeljax
| #
Почему Нимесил запрещён в некоторых странах? https://x.com/DeyanetKrmv/status/1901394421708452176
Silver_Pioneer
| #
https://inderjeet.co.uk/hello-world Где учиться бизнесу?
mtsekaterinburgsig
| #
мтс домашний интернет
https://www.finceptives.com/employer/24inetrnet-domashnij-ekb-3/
мтс интернет екатеринбург
DonDonfaita
| #
dark markets 2025 https://github.com/darkwebwebsites/darkwebwebsites darknet markets url
Volodyanaf
| #
dark web sites https://darkode-market.com/ – dark web market list
Markcen
| #
Покупка квартиры без рисков? Узнайте подробности https://blok-beton.ru/
FNDaviditabe
| #
darkmarket link https://firstdarkmarket.com/ – dark web drug marketplace
WilliamIRrutty
| #
best darknet markets https://idarknetmarket.com/ – darknet drugs
Terencesam
| #
Спины за регистрацию без депозита с выводом Бонусы за регистрацию
Donaldlaf
| #
dark web market links https://github.com/darkwebmarketslinks/darkwebmarkets dark web link
Toliknaf
| #
darknet market links https://github.com/darknetmarketslist/darknetmarketslist darknet drug links
Pingrutty
| #
darkmarket 2025 https://github.com/darknetmarkets2025/darknetmarketlinks darknet drug store
HoraceHoons
| #
https://shopbankrot.ru/
Dnrtelb
| #
Где заказать диплом по актуальной специальности?
Мы изготавливаем дипломы любой профессии по выгодным ценам. Мы готовы предложить документы техникумов, расположенных в любом регионе России. Вы имеете возможность заказать диплом за любой год, в том числе документы старого образца. Дипломы и аттестаты выпускаются на бумаге высшего качества. Это дает возможность делать государственные дипломы, которые не отличить от оригинала. Документы будут заверены необходимыми печатями и штампами. Всегда стараемся поддерживать для заказчиков адекватную политику тарифов. Важно, чтобы документы были доступны для большинства граждан. diplom5.com/kupit-diplom-v-kirove-6
"https://wiki.lawpret.com/index.php?title=Exsrocket.ru_85w"
| #
Обменял биткоин на Сбербанк – все супер!
“https://ashwoodvalleywiki.com/index.php?title=User:TeresitaDqb”
MarkNOlaf
| #
darknet websites https://kingdom-darkmarketplace.com – darkmarket 2025
"https://elearnportal.science/wiki/User:WyattKeenum6"
| #
Купил биткоин – все быстро,
удобно и надежно.
“https://antoinelogean.ch/index.php?title=Benutzer:GarrettMayon7”
DonDonfaita
| #
darkmarket link https://github.com/darkwebwebsites/darkwebwebsites darknet markets links
Volodyanaf
| #
dark web drug marketplace https://darkfoxdarkweb.com/ – darkmarket
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://garantsteel.ru/
HoraceHoons
| #
https://shopbankrot.ru/
FNDaviditabe
| #
darknet markets onion address https://kingdom-marketplace.com/ – dark market url
"https://msgwiki.msgeducation.com/index.php/Exsrocket.ru_88J"
| #
Лучший курс на обмен биткоинов – рекомендую!
“https://funsilo.date/wiki/Exsrocket.ru_37n”
WilliamIRrutty
| #
dark markets https://darknetmarketsonion.shop/ – darknet markets
Donaldlaf
| #
dark market onion https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets onion
Pingrutty
| #
darknet sites https://github.com/darknetmarkets2025/darknetmarketlinks dark web drug marketplace
Toliknaf
| #
darknet market links https://github.com/darknetmarketslist/darknetmarketslist darknet site
Kxyufaita
| #
darknet marketplace https://cannahomemarket.link/ – dark websites
mtsekaterinburgsig
| #
мтс тарифы екатеринбург
https://www.referall.us/employer/24inetrnet-domashnij-ekb-2/
мтс подключение екатеринбург
MarkNOlaf
| #
darknet websites https://kingdom-darkmarketplace.com – darknet websites
Dark413
| #
https://www.graemestrang.com/?page_id=11 Как монетизировать бизнес в бизнесе?
DonDonfaita
| #
dark websites https://github.com/darkwebwebsites/darkwebwebsites dark market url
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://interpraiz.ru/
Volodyanaf
| #
darknet marketplace https://darkwebversus.com/ – darknet drug store
Pingrutty
| #
dark market url https://github.com/darknetmarkets2025/darknetmarketlinks darkmarket link
FNDaviditabe
| #
darknet market https://kingdom-marketplace.com/ – darknet markets links
Donaldlaf
| #
darknet links https://github.com/darkwebmarketslinks/darkwebmarkets darknet site
WilliamIRrutty
| #
tor drug market https://kingdommarketdarknet.com/ – darknet markets onion address
whois7.ru
| #
Собрание сочинений в 7 томах // Казино Руайаль / Перевод с англ.
Казино “Рояль” / Перевод с англ. ↑ A
buyer’s guide to casino collectibles (неопр.).
my blog post … whois7.ru
Toliknaf
| #
darknet sites https://github.com/darknetmarketslist/darknetmarketslist bitcoin dark web
Kxyufaita
| #
darknet links https://cannahome-darkmarket-online.com/ – dark web drug marketplace
MarkNOlaf
| #
best darknet markets https://cannahomedarknetdrugstore.com/ – darkmarket url
DonDonfaita
| #
darknet drug links https://github.com/darkwebwebsites/darkwebwebsites darknet links
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://dmitrov-klimat.ru/
Pingrutty
| #
dark market 2025 https://github.com/darknetmarkets2025/darknetmarketlinks darknet markets links
Volodyanaf
| #
dark web market urls https://darkwebversus.com/ – dark web marketplaces
FNDaviditabe
| #
darknet marketplace https://onion-dark-market.shop/ – darknet site
Donaldlaf
| #
dark web market links https://github.com/darkwebmarketslinks/darkwebmarkets darknet links
Ronaldbix
| #
Bu burcl?r pula daha meyillidir https://www.pinterest.com/pin/957366833290413195
WilliamIRrutty
| #
darknet drug market https://darknetmarketsonion.shop/ – darknet markets onion
Toliknaf
| #
dark web marketplaces https://github.com/darknetmarketslist/darknetmarketslist darknet market
JohnnyGibra
| #
Ищете качественный ремонт квартиры? Мы предлагаем полный спектр услуг – от капитального ремонта до дизайнерской отделки. Профессиональные сантехники, опытные строители и отделочники помогут вам отремонтировать жильё любого типа: однокомнатный, двухкомнатный или четырехкомнатный. Мы проведем демонтаж, перепланировку и замену мебели, а также улучшим интерьер вашей кухни или ванной. Гарантируем доступные цены и высокое качество выполнения работ. Звоните, чтобы обсудить проект и узнать стоимость услуг: квартиры ремонт под ключ
Kxyufaita
| #
darknet sites https://cyberdarkmarkets.com/ – darkmarkets
prodvijenie saitov v moskve_eaol
| #
сео продвижение цена в месяц http://www.prodvizhenie-sajtov-v-moskve224.ru/ .
lkjhCype
| #
http://devec.ru/Joom/pgs/filmu_144.html карантин у входа в рай 9 букв
Terencesam
| #
Бонус с выводом без депозита bonus casino
Pingrutty
| #
dark web link https://github.com/darknetmarkets2025/darknetmarketlinks darknet drug store
WolfWarden
| #
Новые подходы https://constcourt.tj/en/2020/01/30/working-visit-to-new-york-city-21-09-2017/ в бизнесе.
HarryMup
| #
Секреты красоты и здоровья https://magiclady.kyiv.ua в одном месте! Полезные советы по уходу, тренды моды, спорт, правильное питание и психология уверенности. Узнайте, как выглядеть и чувствовать себя великолепно!
JeffreySwicy
| #
Ваш гид по стилю https://magictech.com.ua красоте и жизни! Женский журнал о модных трендах, секретах молодости, психологии, карьере и личных отношениях.
MarkNOlaf
| #
darknet drug store https://kingdom-darkmarketplace.com – darkmarket list
Antoniocen
| #
Лучший женский журнал https://modam.com.ua онлайн! Мода, стиль, уход за собой, фитнес, кулинария, саморазвитие и лайфхаки. Полезные советы и вдохновение для современной женщины, которая стремится к лучшему!
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://petrovsk05.ru/
DonDonfaita
| #
darknet links https://github.com/darkwebwebsites/darkwebwebsites darknet markets
Volodyanaf
| #
dark web market links https://darkode-market.com/ – darknet markets url
Donaldlaf
| #
darkmarket list https://github.com/darkwebmarketslinks/darkwebmarkets darknet market list
FNDaviditabe
| #
darknet sites https://onion-dark-market.shop/ – dark web market urls
WilliamIRrutty
| #
darknet markets url https://kingdomdarkmarketonline.com/ – dark web sites
Toliknaf
| #
best darknet markets https://github.com/darknetmarketslist/darknetmarketslist best darknet markets
Pingrutty
| #
darknet market links https://github.com/darknetmarkets2025/darknetmarketlinks darknet markets url
Kxyufaita
| #
darknet markets https://cannahome-darkmarket-online.com/ – tor drug market
1win_pzPl
| #
1win,com 1win717.ru .
1win_wspa
| #
игра 1вин https://www.1win818.ru .
1win_nqMr
| #
1 win официальный https://1win819.ru .
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные рекомендации — https://profil-orel.ru/
MarkNOlaf
| #
darknet drugs https://dark-web-versus.com – onion dark website
DonDonfaita
| #
best darknet markets https://github.com/darkwebwebsites/darkwebwebsites darkmarket url
Jariormns
| #
Где приобрести диплом специалиста?
Мы предлагаем выгодно приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, штампами, подписями. Документ способен пройти лубую проверку, даже с использованием профессионального оборудования. Достигайте цели быстро и просто с нашей компанией. Приобрести диплом любого университета! sportpassionhub.com/read-blog/4472_diplom-povara-3-razryada.html
VictorSef
| #
Дизайн-проект под ключ – это удобно! Узнайте, как воплотить свои идеи в реальность в публикации ремонт квартиры с дизайн проектом под ключ, где специалисты объясняют все этапы создания гармоничного и функционального пространства.
Volodyanaf
| #
dark web link https://darkmarketpremium.com/ – dark markets
DavidSuh
| #
генератор голоса онлайн бесплатно
VincentVot
| #
Современный женский онлайн https://viplady.kyiv.ua журнал для тех, кто хочет большего! Всё о моде, косметике, фитнесе, питании, психологии, карьере и отношениях. Узнавайте новое, вдохновляйтесь и воплощайте мечты в реальность!
Terryaddip
| #
Онлайн-журнал для женщин https://one-lady.com которые хотят быть стильными и успешными! Последние тренды, секреты красоты, уход за собой, здоровье, психология и карьера. Будьте в курсе новинок и наслаждайтесь жизнью!
AndrewJoict
| #
Ваш женский путеводитель https://topwoman.kyiv.ua по красоте, стилю и саморазвитию! Полезные советы, модные тенденции, лайфхаки по уходу за собой, психология, отношения и вдохновение.
Donaldlaf
| #
dark web market links https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets url
Anthonydum
| #
Выявлен единственный напиток, который спасает волосы https://x.com/Fariz418740/status/1901556598939468146
Pingrutty
| #
dark web market https://github.com/darknetmarkets2025/darknetmarketlinks darknet drugs
FNDaviditabe
| #
dark web link https://onion-dark-market.shop/ – darknet markets links
WilliamIRrutty
| #
dark websites https://kingdommarketdarknet.com/ – darknet markets onion
Toliknaf
| #
darkmarket list https://github.com/darknetmarketslist/darknetmarketslist darknet links
Kxyufaita
| #
darknet sites https://darkfoxmarketplace.com/ – darkmarket url
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://lead-stone.ru/
Silver_Protocol
| #
https://www.kaeshammer.ch/bouquets/ Нужны реальные кейсы по бизнесу.
EdwardBairl
| #
Zajrzalem na serwis unit okulistyczny i momentalnie trafilem na balagan. Interfejs przestarzaly, nawigacja zawila, a tresci niezweryfikowane. Pewne artykuly sa sprzeczne z soba nawzajem, a potrzebnych informacji brakuje. Bledy na sekcjach, dzialanie opoznione, a obsluga ignoruje wiadomosci. Jesli chcesz miec dokladne informacje – omijaj tej platformy.
MarkNOlaf
| #
darknet drug market https://drdarkfoxmarket.com – darknet drug links
komputerny-ekspertcaree
| #
Подробнее в обозревательном журнале компьютерный эксперт здесь
DonDonfaita
| #
bitcoin dark web https://github.com/darkwebwebsites/darkwebwebsites darkmarket url
1win_snPl
| #
1win,com http://www.1win717.ru .
StevenHip
| #
посмотреть в этом разделе https://vodkabet.io
1win_htpa
| #
1вин официальный мобильная 1вин официальный мобильная .
prodvijenie saitov v moskve_pxsl
| #
рассчитать стоимость раскрутки сайта http://www.prodvizhenie-sajtov-v-moskve225.ru .
beton ot proizvoditelya_gvOa
| #
цена бетона минск цена бетона минск .
Pingrutty
| #
darknet drug store https://github.com/darknetmarkets2025/darknetmarketlinks darkmarkets
1win_rfMr
| #
бк 1win https://1win819.ru/ .
FNDaviditabe
| #
dark market link https://mydarkmarketlink.com/ – dark market link
Donaldlaf
| #
darknet market lists https://github.com/darkwebmarketslinks/darkwebmarkets dark market onion
TimothyErumb
| #
Портал для родителей https://rodkom.org.ua всё о воспитании, развитии и здоровье детей! Полезные советы, лайфхаки, педиатрические рекомендации, семейная психология и идеи для досуга. Растите счастливых и здоровых детей вместе с нами!
WilliamIRrutty
| #
dark web market list https://kingdommarketdarknet.com/ – darknet markets
RichardAbinc
| #
Автомобильный портал https://kakavto.com всё, что нужно знать о машинах! Новинки автопрома, технологии, электрокары, автоспорт, автострахование и советы по ремонту. Следите за трендами автоиндустрии!
Ramirocep
| #
Советы для родителей https://agusha.com.ua в одном месте! Развитие ребёнка, воспитание, здоровье, питание, семейные отношения и идеи для досуга. Полезные статьи, лайфхаки и экспертные мнения помогут вам в заботе о малыше!
Terrybaway
| #
kra30.cc – kra30.cc, kra29.cc
Toliknaf
| #
darknet markets url https://github.com/darknetmarketslist/darknetmarketslist darkmarket list
Kxyufaita
| #
darknet markets onion https://kingdomdarknetdrugstore.com/ – bitcoin dark web
TimothyHok
| #
Единственный знак зодиака, который не могут раскусить астрологи
https://x.com/CantrellAl79925/status/1901597622399381669
MarkNOlaf
| #
darknet market links https://kingdomdarkmarketplace.com – darkmarket link
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://koncent-n.ru/
DonDonfaita
| #
dark market https://github.com/darkwebwebsites/darkwebwebsites darkmarket 2025
Pingrutty
| #
darknet market list https://github.com/darknetmarkets2025/darknetmarketlinks darknet sites
FNDaviditabe
| #
dark market onion https://kingdom-marketplace.com/ – dark websites
WilliamIRrutty
| #
darkmarket link https://asap-market-onion.com/ – darknet market lists
Thomaslaf
| #
Предсказания Ванги на 2025 год: правда или миф?
https://x.com/Ruslansavalv/status/1901612556772409580
Diplomi_nhKt
| #
Приобрести диплом ВУЗа!
Мы можем предложить документы университетов, которые расположены в любом регионе России.
asxdiplommy.com/attestat-ob-okonchanii-9-klassa-kupit
Donaldlaf
| #
darknet markets links https://github.com/darkwebmarketslinks/darkwebmarkets darkmarket url
Kxyufaita
| #
dark markets 2025 https://cannahome-darkmarket-online.com/ – dark web marketplaces
DanielDub
| #
купить права в москве – получить права в москве, купить права
Toliknaf
| #
dark web market list https://github.com/darknetmarketslist/darknetmarketslist darknet drug market
MarioEvove
| #
Актуальные новости Украины https://vesti.in.ua политика, экономика, спорт, культура и общество! Оперативная информация, эксклюзивные материалы, репортажи и аналитика. Следите за событиями в режиме реального времени!
CharlesTrexy
| #
Hype casino промокод бонусы на депозит – Покерок промокод бонусы на депозит, Бонус код Leon при регистрации
ArthurCak
| #
Главные новости дня https://mostmedia.com.ua политика, экономика, общество, спорт и культура! Оперативные события, аналитика, мнения экспертов и репортажи. Читайте актуальные новости Украины и мира в одном месте!
Jameslat
| #
Медицинский онлайн-журнал https://medicalanswers.com.ua ваш личный консультант по здоровью! Полезные статьи, советы врачей, профилактика, диагностика, лечение и здоровый образ жизни.
MarkNOlaf
| #
darkmarket link https://dark-web-versus.com – darknet links
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://a-prom59.ru/
Terencesam
| #
Бездепозитные бонусы за регистрацию Играть игровые автоматы на деньги
Hacker2025
| #
Плюсы и https://cobsamex.net/inversion/educacion-garantizada/ минусы разных подходов к бизнесу.
Pingrutty
| #
dark web marketplaces https://github.com/darknetmarkets2025/darknetmarketlinks tor drug market
DonDonfaita
| #
darknet markets 2025 https://github.com/darkwebwebsites/darkwebwebsites darknet drugs
FNDaviditabe
| #
darkmarket list https://darkmarketdarkfox.com/ – darkmarket
WilliamIRrutty
| #
dark web market list https://idarknetmarket.com/ – darknet drugs
Storm199
| #
Как реализовали финансов в компании: https://krovlyaug.ru
Kxyufaita
| #
darknet markets links https://cyberdarkmarkets.com/ – darkmarket
Donaldlaf
| #
dark websites https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets onion address
MarkNOlaf
| #
darknet links https://kingdom-darkmarketplace.com – darkmarket url
LarryNek
| #
Доброго!
Вам нужен надежный способ для регистрации на онлайн-платформах? Виртуальный номер навсегда – это идеальный выбор. Он работает в любых условиях. https://www.sport-weekend.com/kogda-neobhodim-virtualnyj-nomer-dlja-chatgpt.htm Купить постоянный виртуальный номер – это безопасно и выгодно. Подключение доступно для каждого. Оцените удобство современных технологий!
Постоянный виртуальный номер для смс – это удобное решение для получения сообщений без привязки к SIM-карте. Он легко подключается. Купить виртуальный номер для смс навсегда – это быстро и удобно. Подключение не займет много времени. Получайте сообщения без ограничений!
купить виртуальный номер для смс навсегда, виртуальный номер, купить виртуальный номер для смс навсегда
Удачи и хорошей связи!
Toliknaf
| #
darkmarket list https://github.com/darknetmarketslist/darknetmarketslist darknet markets onion
Light419
| #
Как устроен механизм финансов? https://allealavto.ru.
GeraldFed
| #
Сайт для женщин https://bbb.dp.ua всё о моде, красоте, здоровье, отношениях и саморазвитии! Читайте полезные советы, следите за трендами, вдохновляйтесь и наслаждайтесь гармоничной жизнью!
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://stroyramen.ru/
Pingrutty
| #
onion dark website https://github.com/darknetmarkets2025/darknetmarketlinks dark markets 2025
Robertwef
| #
Juega sin limites! https://amargordediciones.com ofrecen altas cuotas, pagos rapidos y anonimato. ?Compara casas de apuestas populares, elige metodos de apuestas convenientes y disfruta de la emocion!
baravaca
| #
Apuestas deportivas casa de apuesta internacional plataformas con licencia, cuotas favorables, variedad de deportes y pagos rapidos. ?Consulta la valoracion de las mejores casas de apuestas y elige la tuya!
FNDaviditabe
| #
darknet markets url https://darkmarketdarkfox.com/ – dark web market links
WilliamIRrutty
| #
bitcoin dark web https://kingdomdarkmarketonline.com/ – darknet market lists
DonDonfaita
| #
dark markets https://github.com/darkwebwebsites/darkwebwebsites darkmarket 2025
Kxyufaita
| #
darknet markets links https://cannahomemarket.link/ – dark market 2025
JamesEvede
| #
Odwiedzilem na brzeznica 39-207 i szybko przekonalem sie, ze to jest kompletnie bez sensu. Serwis jest wypelniona banerami, interfejs koszmarny, a tresci sa niskiej jakosci. Bug’i na podstronach, przekierowania prowadza donikad, a obsluga klienta nie odpowiadaja uzytkownikow. Czuc, ze nikt sie tym nie zajmowal i nie byl aktualizowany od bardzo dawna. Jesli zalezy ci na rzetelnych informacjach i latwosci obslugi – nawet nie wchodz.
MarkNOlaf
| #
dark market link https://kingdom-darkmarketplace.com – bitcoin dark web
Donaldlaf
| #
darknet drugs https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets links
beton ot proizvoditelya_asma
| #
бетон с доставкой цена за 1 м3 http://www.beton-lot.ru/ .
Pingrutty
| #
dark web drug marketplace https://github.com/darknetmarkets2025/darknetmarketlinks darknet markets 2025
Toliknaf
| #
dark web market list https://github.com/darknetmarketslist/darknetmarketslist darknet markets onion
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://ash-stroymarket.ru/
FNDaviditabe
| #
darknet drugs https://firstdarkmarket.com/ – dark market url
WilliamIRrutty
| #
darknet markets 2025 https://darknetmarketsonion.shop/ – darknet drug store
PhantomV9
| #
https://myasnayatarelka.ru Мемы про финансы.
ErnestLer
| #
покердом вход
Kxyufaita
| #
dark market onion https://kingdomdarknetdrugstore.com/ – dark market onion
GeorgeTom
| #
Source:
https://sputniktv-msk.ru/
Bruceunoff
| #
Dobite a preskumajte bobotov-kuk.com a pocitite majestatnost hor! Najvyssi vrch Ciernej Hory (2523 m) ponuka ohromujuce panoramy, vzrusujuce trasy a nezabudnutelne emocie. Zistite najlepsie lezecke cesty a tipy na bezpecny vylet!
DonDonfaita
| #
darkmarkets https://github.com/darkwebwebsites/darkwebwebsites darkmarket 2025
Sidneytow
| #
Otkrijte most https://www.durdevica-tara-bridge.com – jedan od najlepsih mostova u Evropi! Jedinstvena gradevina iznad kanjona Tare zadivljuje svojom razmjerom i panoramskim pogledom. Najbolje rute, izleti i savjeti za putnike!
Malcomvag
| #
Kaufen Sie immobilien in montenegro – Ihr Zuhause am Meer oder in den Bergen! Tolle Angebote, Villen, Wohnungen und Apartments. Informieren Sie sich uber die besten Lagen, Preise, rechtlichen Feinheiten und Investitionsmoglichkeiten!
ThomasVep
| #
Спасибо за качественный сервис, мама была в восторге от букета!
доставка цветов в томске
MarkNOlaf
| #
darkmarket link https://kingdomdarkmarketplace.com – darknet markets 2025
Pingrutty
| #
darknet drugs https://github.com/darknetmarkets2025/darknetmarketlinks darknet site
Donaldlaf
| #
dark market list https://github.com/darkwebmarketslinks/darkwebmarkets darkmarket url
FNDaviditabe
| #
dark market link https://onion-dark-market.shop/ – bitcoin dark web
WilliamIRrutty
| #
dark market 2025 https://kingdommarketdarknet.com/ – dark market list
Toliknaf
| #
darknet websites https://github.com/darknetmarketslist/darknetmarketslist dark web markets
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные рекомендации — https://optima-family.ru/
Sazrwyn
| #
Мы изготавливаем дипломы любой профессии по выгодным тарифам. Стоимость будет зависеть от выбранной специальности, года получения и университета. Стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас важно, чтобы дипломы были доступными для большого количества наших граждан. купить диплом с отличием
lkjhCype
| #
http://alexey-savrasov.ru/records/articles/filmu_o_poiske_sokrovish_besplatno.html фильмы с высоким рейтингом скачать на телефон
Kxyufaita
| #
dark web markets https://kingdomdarknetdrugstore.com/ – dark markets
MarkNOlaf
| #
tor drug market https://drdarkfoxmarket.com – darknet markets
obzor-pricelovcaree
| #
Подробнее в блоге обзоров прицелов и другой оптической техники ссылка
DonDonfaita
| #
dark web market urls https://github.com/darkwebwebsites/darkwebwebsites darknet websites
https://cryptolake.online/crypto2
| #
my site https://cryptolake.online/crypto2
Pingrutty
| #
darknet market https://github.com/darknetmarkets2025/darknetmarketlinks darknet market list
Terencesam
| #
no deposit casino no deposit casino
Master8528
| #
Подкаст о https://lombardizumrud.ru финансах.
CharlesLar
| #
Sakupon an Salog Tara Tara canyon rafting an pinakamarahay na pag-rafting sa Montenegro! Pag-raft sa saro sa pinakahararom na mga kanyon sa Europa, an pinakadalisay na tubig asin naturalesa kan Durmitor. An perpektong pakikipagsapalaran para sa mga mahilig sa luwas!
KennethSof
| #
Enjoy fun at Durdevica Tara bridge extreme rafting, kayaking, boating and camping in picturesque places. The perfect place for active recreation and immersion in wild nature!
FNDaviditabe
| #
darknet sites https://firstdarkmarket.com/ – darkmarket 2025
Light_Protocol
| #
Мне самому помог этот https://glykas.com.gr/2017/09/27/highlights-from-hong-kongs-art-central/ ресурс по бизнесу.
WilliamIRrutty
| #
dark web market urls https://kingdomdarkmarketonline.com/ – darkmarket list
Donaldlaf
| #
darknet drug store https://github.com/darkwebmarketslinks/darkwebmarkets darknet market list
Mariotox
| #
Хотите списать долги? списание долгов помощь в сложных финансовых ситуациях. Освободитесь от кредитов, штрафов и задолженностей. Узнайте, как начать процедуру прямо сейчас!
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://eco-construct.ru/
Kxyufaita
| #
darknet drugs https://cyberdarkmarkets.com/ – darknet markets onion
NightX6
| #
Успешные кейсы применения финансов: https://triumph-uk.ru
Toliknaf
| #
bitcoin dark web https://github.com/darknetmarketslist/darknetmarketslist darkmarket url
Cazrowa
| #
Приобрести диплом о высшем образовании!
Мы предлагаем документы институтов, расположенных в любом регионе России. Можно купить диплом за любой год, включая сюда документы старого образца. Документы выпускаются на бумаге высшего качества: dlja-pohudenija.ru/news/gde_kupit_attestat_za_9_klass_bustro_i_nadezghno.html
MarkNOlaf
| #
dark web drug marketplace https://kingdomdarkmarketplace.com – bitcoin dark web
Frankcrica
| #
Здравствуйте, жители Мариуполя! Ищите новые знакомства? Наш сайт знакомств 18+ — это лучшее место для того, чтобы начать. Вас ждут красивые и интересные девушки, с которыми вы сможете обсудить всё на свете. Присоединяйтесь и открывайте новые горизонты общения https://t.me/prostitutki_mariupol_indi
Frankcrica
| #
Друзья, представляем вам новый сайт, который станет настоящей находкой для всех, кто ищет интересное времяпрепровождение с девушками 18+. Мариуполь теперь может похвастаться площадкой, где каждая встреча станет незабываемой и принесет море удовольствия и эмоций, заходите и любите каждый момент: проститутки мариуполь тг
Pingrutty
| #
darknet links https://github.com/darknetmarkets2025/darknetmarketlinks darknet markets links
DonDonfaita
| #
dark web link https://github.com/darkwebwebsites/darkwebwebsites dark market list
Jamesdit
| #
Лучшие девушки Донецка ждут вас на нашем сайте, где вы сможете выбрать наилучший вариант для отдыха. Не упустите шанс сделать свою жизнь ярче и насыщеннее, предоставив себе возможность насладиться обществом привлекательной дамы. Приходите и выбирайте https://t.me/prostitutki_donetsk_individualki
FNDaviditabe
| #
darknet markets links https://darkmarketdarkfox.com/ – dark web sites
WilliamIRrutty
| #
dark web sites https://asap-market-onion.com/ – darknet markets 2025
1win_abpa
| #
1win на телефон 1win на телефон .
1win_kg_zwel
| #
1win. com 1win. com .
1win_kg_qrpa
| #
1win букмекер 1win букмекер .
Kxyufaita
| #
dark web drug marketplace https://darkfoxmarketplace.com/ – dark market
Donaldlaf
| #
best darknet markets https://github.com/darkwebmarketslinks/darkwebmarkets darkmarket link
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://mastera-krovli.ru/
MarkNOlaf
| #
tor drug market https://cannahomedarknetdrugstore.com/ – dark web market list
Toliknaf
| #
darknet drug links https://github.com/darknetmarketslist/darknetmarketslist darknet markets
Aaroncon
| #
Неожиданный способ есть финики превращает их в природное лекарство
https://x.com/SebiBilalova/status/1901774516755309011
Pingrutty
| #
dark web link https://github.com/darknetmarkets2025/darknetmarketlinks darkmarket list
FNDaviditabe
| #
darknet site https://kingdom-marketplace.com/ – darknet markets onion address
Rabyitabe
| #
darknet markets links https://github.com/darknetmarketlinks2025/darknetmarkets darknet sites
WilliamIRrutty
| #
dark markets https://asap-market-onion.com/ – darknet market
Kxyufaita
| #
darknet markets links https://cannahome-darkmarket-online.com/ – darknet markets 2025
DonDonfaita
| #
darkmarket link https://github.com/darkwebwebsites/darkwebwebsites dark web marketplaces
StormPioneer
| #
Перспективы финансов: https://123inv.ru
Dragon_Striker
| #
Мнение специалистов о бизнесе: https://mueenahmed.com/?p=4
MarkNOlaf
| #
darknet sites https://kingdom-darkmarketplace.com – dark market
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://mirsnaryagi.ru/
BlueV9
| #
Успешные https://1pzk.ru кейсы применения финансов.
1win_aypa
| #
1 вин официальный сайт вход https://www.1win820.ru .
1win_kg_hxel
| #
1win кейсы 1win821.ru .
Donaldlaf
| #
best darknet markets https://github.com/darkwebmarketslinks/darkwebmarkets darknet drugs
FNDaviditabe
| #
darknet drugs https://kingdom-marketplace.com/ – dark market link
1win_kg_uopa
| #
1 вин скачать https://1win808.ru .
Pingrutty
| #
darknet sites https://github.com/darknetmarkets2025/darknetmarketlinks darkmarket url
WilliamIRrutty
| #
darkmarket https://kingdommarketdarknet.com/ – dark market url
Toliknaf
| #
darknet websites https://github.com/darknetmarketslist/darknetmarketslist darknet drug store
Kxyufaita
| #
darknet markets https://cannahome-darkmarket-online.com/ – darknet drug store
Rabyitabe
| #
dark market list https://github.com/darknetmarketlinks2025/darknetmarkets bitcoin dark web
MarkNOlaf
| #
dark market 2025 https://kingdom-darkmarketplace.com – dark market url
Lorenzowip
| #
Been relying on them for years, and they never disappoint.
get lisinopril without dr prescription
Comprehensive side effect and adverse reaction information.
DonDonfaita
| #
dark web marketplaces https://github.com/darkwebwebsites/darkwebwebsites dark markets 2025
Sykaaaa casino
| #
Woah! I’m really enjoying the template/theme of this website.
It’s simple, yet effective. A lot of times it’s hard to get that “perfect balance” between usability and visual appeal.
I must say you have done a awesome job with this. Also, the
blog loads extremely fast for me on Safari. Outstanding Blog!
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://kama-stroi.ru/
FNDaviditabe
| #
darknet market list https://onion-dark-market.shop/ – dark web marketplaces
WilliamIRrutty
| #
dark market url https://asap-market-onion.com/ – darknet markets links
Jamesdit
| #
На сайте для досуга 18+ вас ждут настоящие сокровища. Каждая девушка, представленная здесь, готова сделать все, чтобы ваш вечер стал незабываемым. Великолепные встречи, интересные разговоры и время, наполненное страстью — это то, что ждёт вас. Не упустите возможность провести вечер с яркими личностями, которые превратят ваши мечты в реальность https://t.me/prostitutki_donetsk_individualki
Pingrutty
| #
darkmarket link https://github.com/darknetmarkets2025/darknetmarketlinks darknet drug links
dandtautobody.comensub
| #
Elevate your driving experience with D&T Auto Body’s premium tuning treatments. Our team of professionals specializes in upgrading your vehicle to unleash its true capability . Whether you seek swiftness or chic , our bespoke solutions provide comprehensive results. Discover the change at https://dandtautobody.com/ and see how we can turn your car into a masterpiece .
Terencesam
| #
Top casino с выводом Бездепозитные бонусы за регистрацию
Kxyufaita
| #
darkmarket link https://kingdomdarknetdrugstore.com/ – dark websites
Donaldlaf
| #
dark web marketplaces https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets links
Cazrnnn
| #
Здравствуйте!
Без наличия диплома сложно было продвигаться вверх по карьере. Сейчас этот важный документ не дает никаких гарантий, что получится получить престижную работу. Более важное значение имеют практические навыки специалиста и его постоянный опыт. Именно из-за этого решение о покупке диплома можно считать целесообразным. Приобрести диплом об образовании sg.getbb.ru/viewtopic.php?f=8&t=4240
MarkNOlaf
| #
darknet marketplace https://dark-web-versus.com – darknet links
Shadow393
| #
Приветствую. https://activefond.ru Подскажите, где посмотреть информацию о финансах?
Sazrzma
| #
Приветствую!
Приобрести диплом университета по невысокой цене можно, обратившись к проверенной специализированной фирме. Заказать диплом о высшем образовании: kupit-diplom24.com/kupit-diplom-astraxan-6/
Toliknaf
| #
onion dark website https://github.com/darknetmarketslist/darknetmarketslist darknet site
FNDaviditabe
| #
darknet links https://onion-dark-market.shop/ – darknet drug links
Rabyitabe
| #
best darknet markets https://github.com/darknetmarketlinks2025/darknetmarkets darknet markets 2025
WilliamIRrutty
| #
darknet drugs https://kingdommarketdarknet.com/ – darknet drug market
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://abrikos-krsk.ru/
Kxyufaita
| #
best darknet markets https://cyberdarkmarkets.com/ – darknet drug links
DonDonfaita
| #
darknet marketplace https://github.com/darkwebwebsites/darkwebwebsites dark web link
OmegaZ3
| #
История становления https://www.peretyazhka-mebeli-v-novosibirske-154.ru/ бизнеса.
Pingrutty
| #
darkmarket link https://github.com/darknetmarkets2025/darknetmarketlinks darknet market links
MarkNOlaf
| #
darkmarket 2025 https://cannahomedarknetdrugstore.com/ – darknet drug store
Donaldlaf
| #
best darknet markets https://github.com/darkwebmarketslinks/darkwebmarkets dark web market list
internet provaideri_fzSr
| #
интернет в москве провайдеры выгодный тариф интернет в москве провайдеры выгодный тариф .
FNDaviditabe
| #
dark web drug marketplace https://firstdarkmarket.com/ – darknet marketplace
internet provaideri_jepr
| #
домашний интернет и телевидение в москве домашний интернет и телевидение в москве .
lkjhCype
| #
http://kib-net.ru/news/pgs/filmu_pro_piratov__zahvatuvaushiy_mir_morskih_priklucheniy.html авито тверь снять дом посуточно
Alpha203
| #
Пример из https://cm-rf.ru практики: Финансы.
WilliamIRrutty
| #
dark market onion https://idarknetmarket.com/ – darknet drugs
beton ot proizvoditelya_vspl
| #
1 куб бетона цена с доставкой 1 куб бетона цена с доставкой .
Toliknaf
| #
darkmarket link https://github.com/darknetmarketslist/darknetmarketslist darknet site
dostavka alkogolya 24_yvkl
| #
алкоголь 24 часа доставка алкоголь 24 часа доставка .
Kxyufaita
| #
darknet drugs https://cyberdarkmarkets.com/ – darknet market list
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные рекомендации — https://smudsmetall.ru/
Rabyitabe
| #
darknet market list https://github.com/darknetmarketlinks2025/darknetmarkets dark market
MarkNOlaf
| #
darknet drug links https://kingdomdarkmarketplace.com – dark market url
Brianpen
| #
Эти четыре знака Зодиака накроет волна счастья уже на этой неделе
https://www.pinterest.com/pin/957366833290452816/
Pingrutty
| #
darknet links https://github.com/darknetmarkets2025/darknetmarketlinks darknet markets 2025
Iron452
| #
ТОП-10 ресурсов по финансам: https://centrdeklaraciy22.ru
DonDonfaita
| #
darkmarket https://github.com/darkwebwebsites/darkwebwebsites dark web markets
localtorontomovers
| #
canada movers toronto movers toronto
Lorenzowip
| #
A beacon of trust in international pharmacy services.
buy generic fluoxetine
They always have the newest products on the market.
FNDaviditabe
| #
best darknet markets https://darkmarketdarkfox.com/ – dark websites
JamesMum
| #
цифровые ключи
WilliamIRrutty
| #
dark web sites https://darknetmarketsonion.shop/ – darkmarket link
Donaldlaf
| #
darknet site https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets 2025
Kxyufaita
| #
dark market onion https://cyberdarkmarkets.com/ – tor drug market
Michaelpat
| #
Статьи о ремонте и строительстве https://tvin270584.livejournal.com советы, инструкции и лайфхаки! Узнайте, как выбрать материалы, спланировать бюджет, сделать ремонт своими руками и избежать ошибок при строительстве.
Toliknaf
| #
onion dark website https://github.com/darknetmarketslist/darknetmarketslist darknet markets links
BlakeCot
| #
Ремонт и сантехника https://santekhnik-moskva.blogspot.com пошаговые инструкции, выбор материалов, монтаж систем водоснабжения и отопления, устранение протечек и установка сантехнических приборов. Советы экспертов для качественного ремонта!
MarkNOlaf
| #
darknet drugs https://kingdom-darkmarketplace.com – dark market
Pingrutty
| #
dark web market urls https://github.com/darknetmarkets2025/darknetmarketlinks darknet drug market
JamesPat
| #
в чем разница между seo-агентством и частным специалистом? этот материал поможет вам сделать правильный выбор – https://1st-seo.ru/
Jamesmuh
| #
Новости La Liga
Rabyitabe
| #
darknet markets https://github.com/darknetmarketlinks2025/darknetmarkets dark markets 2025
Займ на карту без отказа
| #
Яндекс помог найти сайт с отличными условиями: микрокредит на карту без отказа доступен каждому с 18 лет. Заполнил заявку, получил одобрение и деньги моментально на карту.
FNDaviditabe
| #
dark market list https://firstdarkmarket.com/ – darkmarket
SteelX5
| #
Секретные методы https://myavto24.ru/ бизнеса.
WilliamIRrutty
| #
dark markets https://darknetmarketsonion.shop/ – tor drug market
Arthursic
| #
Portal big bass bonanza pragmatic play to prawdziwy dramat! Wyglad jest stary, sekcje otwieraja sie wolno, a potrzebnych danych brakuje. Zakladki sa puste, albo sa wypelnione przestarzale dane. Menu chaotyczna, linki sa bledne, a na pytania do wsparcia technicznego wszyscy ignoruja. Jasne jest, ze serwis jest opuszczona i nikogo nie obchodzi. Gdy zalezy Ci na rzetelnych baz danych – szkoda czasu!
Kxyufaita
| #
darknet websites https://cannahomemarket.link/ – darknet markets url
Light750
| #
Смешные случаи в финансах: https://pgl48.ru
FrankTar
| #
Новости Бокса
Dragon504
| #
Какие платформы https://nika42.ru использовать для финансов?
MarkNOlaf
| #
dark web link https://drdarkfoxmarket.com – dark web market list
Donaldlaf
| #
dark web market links https://github.com/darkwebmarketslinks/darkwebmarkets dark web market list
mostbet_ycEa
| #
мостбет авиатор https://mostbet1006.com.kg .
Кэт казино на русском языке
| #
Ищете онлайн-казино с быстрыми выплатами?
Добро пожаловать в Cat Casino! На этой платформе доступны лучшие слоты от ведущих провайдеров, щедрые акции и гарантированные выигрыши!
Кэт игры с высоким RTP.
Какие преимущества
у этого казино?
Большая коллекция слотов от известных студий.
Щедрые предложения на первый депозит.
Быстрые выплаты в любое время суток.
Удобный интерфейс совместим с ПК и смартфонами.
Онлайн-чат оперативно решает вопросы.
Присоединяйтесь к Cat Casino и почувствуйте настоящий
азарт без ограничений!
Ghost_Warden
| #
Друзья, поделитесь опытом по бизнесу! https://furycoins.ru/
Pingrutty
| #
dark web market links https://github.com/darknetmarkets2025/darknetmarketlinks darknet marketplace
Protocol5495
| #
Согласно исследованиям, лучший источник о https://forumlease.ru финансах.
Toliknaf
| #
darkmarket link https://github.com/darknetmarketslist/darknetmarketslist darknet markets onion
TimothyKEx
| #
Нужна машина в прокат? аренда авто анкара быстро, выгодно и без лишних забот! Машины всех классов, удобные условия аренды, страховка и гибкие тарифы. Забронируйте авто в несколько кликов!
PhantomV4
| #
Эксперты https://byh-v.ru рекомендуют эти материалы по финансам.
mostbet_kg_psei
| #
мостбет казино войти mostbet1007.com.kg .
FNDaviditabe
| #
dark market 2025 https://mydarkmarketlink.com/ – darknet market
Terencesam
| #
Бонус за регистрацию игровые автоматы без депозита Игровые автоматы с бездепозитным бонусом за регистрацию с выводом
WilliamIRrutty
| #
darknet markets links https://asap-market-onion.com/ – darkmarket list
mostbet_kg_rrpa
| #
motbet mostbet1008.com.kg .
Rabyitabe
| #
darknet sites https://github.com/darknetmarketlinks2025/darknetmarkets darknet site
OmegaReaper
| #
Инфографика по финансам: https://clover-leasing.ru
Light_Protocol
| #
https://auto-magazine.net/ Шутки про бизнес.
Kxyufaita
| #
darknet market list https://cannahome-darkmarket-online.com/ – darknet websites
DonDonfaita
| #
dark markets 2025 https://github.com/darkwebwebsites/darkwebwebsites darkmarket link
DavidKex
| #
2 знака зодиака, от которых практически невозможно что-либо скрыть
https://pin.it/1l95y2WeH
esports
| #
explosives esports news whores
DarkHunter
| #
Секреты профессионалов в финансах: https://buhgalter161.ru
MarkNOlaf
| #
darknet marketplace https://cannahomedarknetdrugstore.com/ – darkmarket 2025
here
| #
Ready-made custom works here fast, high-quality and inexpensive! We will help with abstracts, term papers, diplomas, essays and other academic assignments. Guaranteed originality, deadlines and 24/7 support!
Shadow_Overlord
| #
Видеоуроки по финансам: https://kredit-taxi.ru
Steel810
| #
Новые подходы https://91j.ru/ в бизнесе.
Pingrutty
| #
dark market url https://github.com/darknetmarkets2025/darknetmarketlinks dark web markets
Bryan Parker
| #
Do you want to experience the luxury tours of Jordan like never before? Then look no further than YOLO Jordan Tours and Travel.
https://www.oneidauniversity.com/
Life Experience Degree
| #
Today, while I was at work, my sister stole my apple ipad and tested to see if it can survive a thirty foot drop, just so she can be a youtube sensation. My apple ipad is now broken and she has 83 views.
https://www.charlestonstateuniversity.com/
Donaldlaf
| #
dark web market https://github.com/darkwebmarketslinks/darkwebmarkets dark web market
Mazrdja
| #
Мы изготавливаем дипломы любых профессий по приятным ценам. Стараемся поддерживать для заказчиков адекватную политику тарифов. Важно, чтобы документы были доступными для большинства наших граждан.
Заказ документа, который подтверждает обучение в университете, – это выгодное решение. Заказать диплом университета: diplomt-v-chelyabinske.ru/kupit-diplom-s-vneseniem-v-reestr-12/
Jariorszs
| #
Где купить диплом по актуальной специальности?
Наши специалисты предлагают быстро и выгодно заказать диплом, который выполнен на оригинальной бумаге и заверен мокрыми печатями, штампами, подписями должностных лиц. Данный документ способен пройти лубую проверку, даже при использовании специального оборудования. Решите свои задачи быстро с нашей компанией.
Купить диплом ВУЗа diplomservis.ru/kupit-attestat-za-11-klass-14/
Iariorcxf
| #
Приобрести документ института можно у нас в столице. [url=http://www.emhudpleie.no/]www.emhudpleie.no[/url]
Sazrjqf
| #
Мы предлагаем дипломы любых профессий по выгодным тарифам.– sharewebsolutions.com/your-business-needs-a-website/.html#comment-15121
Nomad7685
| #
Основы бизнеса для новичков: https://alyonashik.ru/
FNDaviditabe
| #
dark web market list https://onion-dark-market.shop/ – dark web markets
Toliknaf
| #
darknet market list https://github.com/darknetmarketslist/darknetmarketslist dark web drug marketplace
Walker9353
| #
Как https://lombardeconom.ru сделать финансы?
AlphaPioneer
| #
Доступно объяснено о финансах: https://allforb.ru
WilliamIRrutty
| #
dark market link https://kingdommarketdarknet.com/ – darkmarket list
mostbet_jbEa
| #
скачать mostbet скачать mostbet .
Kxyufaita
| #
dark market list https://cyberdarkmarkets.com/ – dark markets
Pioneer4131
| #
Список инструментов по бизнесу: https://myworldland.ru/
Sazrfwi
| #
купить диплом пнипу пермь
DwayneAlire
| #
this premium smm panel
WolfGuardian
| #
Как вам этот ресурс о финансах? https://krasnodar-land.ru
DonDonfaita
| #
dark markets 2025 https://github.com/darkwebwebsites/darkwebwebsites dark markets
Wolf_Slayer
| #
Заинтересовался https://ozcredit.ru финансами. Решение здесь.
MarkNOlaf
| #
darkmarket list https://dark-web-versus.com – dark web marketplaces
mostbet_kg_ysei
| #
мостбет авиатор мостбет авиатор .
beton-moscvich
| #
Нужен качественный бетон? https://beton-moscvich.ru/tovarnii-beton/ быстро, надёжно и по выгодной цене! Прямые поставки от производителя, различные марки, точное соблюдение сроков. Оперативная доставка на стройплощадки, заказ онлайн или по телефону!
Pingrutty
| #
darkmarket list https://github.com/darknetmarkets2025/darknetmarketlinks darknet drugs
Night_Hacker
| #
Как реализовали бизнеса https://dizidom.ru/ в компании.
mostbet_kg_wppa
| #
мосбет казино https://www.mostbet1008.com.kg .
Walker7277
| #
Как устроен механизм финансов? https://ipotekexpert.ru
Lazrded
| #
Диплом университета Российской Федерации!
Без получения диплома сложно было продвинуться вверх по карьерной лестнице. В связи с этим решение о покупке диплома можно считать рациональным. Приобрести диплом о высшем образовании usa.life/read-blog/123118_kupit-diplom.html
FNDaviditabe
| #
dark web link https://kingdom-marketplace.com/ – dark market onion
Donaldlaf
| #
darkmarket url https://github.com/darkwebmarketslinks/darkwebmarkets darkmarket 2025
WilliamIRrutty
| #
dark markets https://kingdommarketdarknet.com/ – darknet markets onion address
Omega_Overlord
| #
Интерактивный курс по бизнесу: https://muntinlupacity.gov.ph/transparency_seal150/
Shadow904
| #
Как выбрать лучшее решение для финансов: https://zaemrub.ru
Toliknaf
| #
darknet market lists https://github.com/darknetmarketslist/darknetmarketslist dark market 2025
AlphaZ1
| #
Мнение специалистов о финансах: https://ipoteka-realtor.ru.
Kxyufaita
| #
dark web market urls https://cannahome-darkmarket-online.com/ – darknet markets onion
CoreyRaiBe
| #
скачать кейсы кс го cs-go.ru/
1winbr
| #
1Win Brasil https://1winbr.com apostas esportivas e cassino online! Altas odds, bonus generosos e ampla variedade de jogos. Aposte em esportes, jogue no cassino e aproveite as melhores promocoes agora mesmo!
Red82
| #
Вдохновляющие истории в https://www.emirates.su/ бизнесе.
Rabyitabe
| #
darknet markets url https://github.com/darknetmarketlinks2025/darknetmarkets darknet market
MarkNOlaf
| #
darkmarkets https://kingdomdarkmarketplace.com – darknet markets
Storm_Master
| #
Давайте обсудим о финансах: https://pkmosfincentr.ru
Pingrutty
| #
darknet drug market https://github.com/darknetmarkets2025/darknetmarketlinks darkmarket link
IronV2
| #
Как https://geografinvest.ru работает механизм финансов?
DonDonfaita
| #
darknet market https://github.com/darkwebwebsites/darkwebwebsites darknet market list
FarukBaile
| #
Хоронил маму, такое горе… “Городская Ритуальная Служба” сделала всё, чтобы проводить достойно. 8-843-227-02-24, г. Казань Советую всем
ThunderV6
| #
Как https://lady3000.ru/ сэкономить на бизнесе.
FNDaviditabe
| #
dark market onion https://mydarkmarketlink.com/ – darknet markets onion
WilliamIRrutty
| #
dark web market list https://idarknetmarket.com/ – darknet market list
lkjhCype
| #
http://www.bf-mechta.ru/wp-content/pages/luchshie_filmu_pro_vikingov_besplatno.html лучший сайт для просмотра фильмов онлайн бесплатно в хорошем качестве без рекламы и регистрации
Cyber_Voyager
| #
Будущее финансов: https://kreditvpermi.ru
ThunderCode
| #
https://szfkspb.ru Как работает механизм финансов?
Donaldlaf
| #
dark market url https://github.com/darkwebmarketslinks/darkwebmarkets darknet markets onion address
Guardian3361
| #
Согласно исследованиям, лучший источник о https://washermdlsettlement.com/menembus-batas-petualangan-seru-judi-online-di-era-digital-2/ бизнесе.
Kxyufaita
| #
dark markets 2025 https://cannahome-darkmarket-online.com/ – dark market list
LanceKew
| #
Prostat x?rc?ngin? qars? hans? qidalar istifad? edilm?lidir? https://x.com/Fariz418740/status/1902037337590108612
Toliknaf
| #
dark web link https://github.com/darknetmarketslist/darknetmarketslist darknet markets onion address
Chrisrat
| #
https://www.businesssoftwarehelp.com/solutioneer/steam-hippo-carpet-cleaning
MarkNOlaf
| #
bitcoin dark web https://cannahomedarknetdrugstore.com/ – onion dark website
Blue_Master
| #
Пример из практики: Финансы – https://magnetinvest.ru
Pingrutty
| #
dark markets https://github.com/darknetmarkets2025/darknetmarketlinks darkmarkets
Scottjam
| #
look these up
Lumi online
CyberSlayer
| #
Авторитетное мнение о бизнесе: http://www.penelopesplace.net/2012/06/07/a-c-petersens/
Terencesam
| #
1000 рублей за регистрацию вывод сразу без вложений Бонус без отыгрыша
Rabyitabe
| #
best darknet markets https://github.com/darknetmarketlinks2025/darknetmarkets dark markets
Shadow571
| #
https://avtolombard-berika.ru Будущее финансов.
Timmymurse
| #
my review here https://web-lumiwallet.com
FNDaviditabe
| #
dark web market list https://mydarkmarketlink.com/ – dark web market urls
Chrisrat
| #
http://www.pagebook.ws/steam-hippo-carpet-cleaning
DonDonfaita
| #
dark web market https://github.com/darkwebwebsites/darkwebwebsites darknet market lists
DragonV1
| #
Где брать актуальные данные о финансах? https://kreditiunas.ru
Alpha209
| #
https://www.auto-russia.com/ Мифы и правда о бизнесе.
Timothydiurn
| #
Новый сайт интимных услуг в Донецке — это возможность испытать лучшие эмоции и наслаждения. С огромным выбором девушек, каждый сможет найти ту, которая наиболее привлекает. Прозрачность и доступность информации о каждую из них позволяет сделать осознанный выбор. Специальные предложения и привилегии для постоянных клиентов станут приятным бонусом для тех, кто ценит качественный сервис – https://donetsk-girl.life/
WilliamIRrutty
| #
darknet links https://asap-market-onion.com/ – dark web sites
1xbetapp
| #
Sports betting with 1xbetapp-br.com/! Huge selection of matches, live bets, generous bonuses and lightning-fast payouts. Play casino, eSports and win at any time!
winbetaz
| #
Welcome to Winbet Casino winbetaz.biz/ a place where luck is on your side! Huge selection of games, bonuses for new players and instant payouts. Try your luck right now!
ThomasVep
| #
Оформление заказа простое и удобное, а цветы невероятной свежести.
доставка цветов томск на дом
Steel_Byte
| #
Памятка для работы с https://time-invest154.ru финансами.
Kxyufaita
| #
dark web market https://cannahome-darkmarket-online.com/ – darknet market
Donaldlaf
| #
dark markets 2025 https://github.com/darkwebmarketslinks/darkwebmarkets dark market link
ShadowZ9
| #
Ошибки новичков в бизнесе: https://bravepatrie.com/spip.php?article1462
Chrisrat
| #
https://www.whizolosophy.com/category/life-s-big-questions/article-essay/transform-your-home-with-professional-cleaning
Warden9891
| #
Ответы на частые вопросы о финансах: https://alfa-constructions.ru
VirtualNek
| #
Здравствуйте!
Хотите пользоваться цифровыми сервисами без риска? Виртуальный номер – это ваш выбор. Он сохраняет конфиденциальность. https://ara-design.com/product/bedroom-table/#comment-11891 Купить постоянный виртуальный номер – значит получить удобный инструмент. Используйте его без ограничений. Оцените безопасность цифровой связи!
Купить виртуальный номер – это оптимальное решение для тех, кто хочет избавиться от лишних звонков. Он идеально подходит для регистрации в разных сервисах. Постоянный виртуальный номер для смс обеспечит вам безопасность и удобство. Получайте сообщения без ограничений. Простота и надежность гарантированы!
постоянный виртуальный номер, постоянный виртуальный номер, Виртуальный номер навсегда
Удачи и хорошей связи!
MarkNOlaf
| #
darknet drug store https://kingdom-darkmarketplace.com – dark web link
Drenaj ychastkov v SPb_gxsr
| #
дренаж участка цена ленинградская дренаж участка цена ленинградская .
Pingrutty
| #
dark market url https://github.com/darknetmarkets2025/darknetmarketlinks dark web market list
Toliknaf
| #
darknet websites https://github.com/darknetmarketslist/darknetmarketslist dark web sites
Dark_Warden
| #
История становления финансов: https://auto-lombard42.ru
Hacker5900
| #
Предлагаю подискутировать https://animedia.xyz/ о бизнесе.
Voyager3321
| #
Реальные примеры применения финансов: https://auto-lombard66.ru
FNDaviditabe
| #
darknet sites https://darkmarketdarkfox.com/ – bitcoin dark web
WilliamIRrutty
| #
dark web market https://darknetmarketsonion.shop/ – darknet links
lkjhCype
| #
http://www.zoolife55.ru/pages/multfilmu_pro_loshadey__chem_oni_pokorili_zriteley_.html фильм поезд идет на восток 1947 смотреть онлайн бесплатно в хорошем качестве
DonDonfaita
| #
darknet drug store https://github.com/darkwebwebsites/darkwebwebsites darknet markets links
Alpha449
| #
5 главных ошибок при работе с бизнесом: http://athletiques.ca/?page_id=2
GhostSlayer
| #
Где https://st-capital.ru брать статистику о финансах?
Dnrtozj
| #
Где купить диплом специалиста?
Мы готовы предложить дипломы любых профессий по невысоким тарифам. Мы готовы предложить документы техникумов, которые находятся в любом регионе РФ. Вы имеете возможность приобрести диплом от любого заведения, за любой год, включая сюда документы СССР. Дипломы и аттестаты делаются на “правильной” бумаге высшего качества. Это дает возможности делать настоящие дипломы, которые не отличить от оригиналов. Они будут заверены необходимыми печатями и штампами. Всегда стараемся поддерживать для покупателей адекватную ценовую политику. Важно, чтобы документы были доступны для большого количества граждан. kupit-diplomyz24.com/kupit-diplom-programmista-5
AlphaX2
| #
Будущее финансов: https://resident-sk.ru
VortexSentinel
| #
Приветствую. Может кто в курсе, где найти информацию о бизнесе? http://smp2guntur-demak.sch.id/forum/
MarkNOlaf
| #
dark web drug marketplace https://dark-web-versus.com – dark web market list
crazymonkeygame
| #
играть crazy monkey бесплатно без регистрации https://crazymonkeygame.com
onewinwin
| #
maximum winnings with onewinwin! Bet on sports, play in the casino, participate in promotions and tournaments. Convenient payments, fast payouts and a welcome bonus for beginners. Join 1win and collect your winnings!
Cyber926
| #
https://homehousecar.ru Вдохновляющие истории в финансах.
RedZ9
| #
Видеоуроки по https://mat-kapitall.ru финансам.
SteelPioneer
| #
Приветствую. https://buildjustly.org/the-launch-of-tech4all-biz/ Подскажите, где посмотреть информацию о бизнесе?
FNDaviditabe
| #
darknet markets https://darkmarketdarkfox.com/ – darknet markets onion
Timothydiurn
| #
Мы предлагаем вам уникальный сайт интимных услуг в Донецке, где каждый найдет свою идеальную спутницу. Удобный интерфейс и возможность фильтровать по параметрам помогут вам выбрать именно то, что вам нужно для приятного вечера: индивидуалки донецк днр
mostbet_kg_hoka
| #
мостбет скачать бесплатно http://mostbet788.ru/ .
Pingrutty
| #
dark web market links https://github.com/darknetmarkets2025/darknetmarketlinks dark market onion
mostbet_yhEa
| #
mostbet kg скачать http://mostbet787.ru .
Donaldlaf
| #
dark market link https://github.com/darkwebmarketslinks/darkwebmarkets dark web markets
1win_szpa
| #
1 win.pro http://1win809.ru .
Phantom239
| #
Перспективы финансов: https://lombardmax.ru
WilliamIRrutty
| #
dark web marketplaces https://idarknetmarket.com/ – dark web marketplaces
Willierex
| #
Существуют моменты в жизни, когда нам кажется, что мы застряли в своем одиночестве. Однако это не приговор, и у нас всегда есть возможность найти приятное общество. Обратите внимание на сайт знакомств с эскортницами, он может стать вашим ключом к новым впечатлениям https://individualki-sochi.life/
Blue_Code
| #
Как сэкономить на финансах: https://garant-zayma.ru
StanleyLot
| #
Заказывайте дезинфекцию помещений у проверенных специалистов с опытом более 10 лет: дезинфекция
Fang8084
| #
Бизнес для стартапов: https://arghealthcare.info/5-things-to-consider-before-buying-a-massage-chair/.
Toliknaf
| #
dark websites https://github.com/darknetmarketslist/darknetmarketslist tor drug market
Kxyufaita
| #
dark web marketplaces https://cyberdarkmarkets.com/ – dark market
Shadow_Byte
| #
Практическое применение финансов: https://ipotekanakvartiru.ru
MarkNOlaf
| #
darknet markets https://dark-web-versus.com – dark market 2025
Storm852
| #
Пытаюсь разобраться в бизнесе. Что посоветуете? http://www.jh1bts.com/freecgi/easybbs/index.cgi?bid=1
Rabyitabe
| #
darknet markets https://github.com/darknetmarketlinks2025/darknetmarkets darknet links
Steel900
| #
Ошибки новичков в финансах: https://souz-zberzaim.ru.
DonDonfaita
| #
best darknet markets https://github.com/darkwebwebsites/darkwebwebsites darknet drug store
Striker8183
| #
С чего начать финансов? https://avto-lombard-stolica.ru
Ghost_Nomad
| #
По данным аналитиков, лучший источник о бизнесе – https://www.skc-max.com/blog/606/
Pingrutty
| #
bitcoin dark web https://github.com/darknetmarkets2025/darknetmarketlinks darknet drug market
WilliamIRrutty
| #
darknet markets links https://darknetmarketsonion.shop/ – bitcoin dark web
Phantom228
| #
Смешные случаи в финансах: https://rostpension.ru
one-1win
| #
gambling without limits http://one-1win.in wide selection of games, high odds, fast payouts and exclusive bonuses. Try your luck in the casino and on bets. Play, win and earn with 1win!
winbkaz
| #
your game, your rules https://winbkaz.biz Sports, eSports, casino, poker and slots – choose and win! Convenient account replenishment, instant withdrawals and cool promotions for new players. Join now!
Donaldlaf
| #
darknet drug store https://github.com/darkwebmarketslinks/darkwebmarkets darknet drugs
Walker4951
| #
Памятка для работы с https://porosnews.id/indo-defence-2022-expo-dan-forum-diikuti-905-perusahaan/ бизнесом.
SilverWalker
| #
Авторитетное мнение о финансах: https://kredit116.ru
Kxyufaita
| #
darkmarket list https://cannahomemarket.link/ – dark web market urls
Toliknaf
| #
tor drug market https://github.com/darknetmarketslist/darknetmarketslist dark market 2025
CyberZ2
| #
Экспертный уровень в финансах: https://megapolistrans.ru
MarkNOlaf
| #
darkmarket 2025 https://drdarkfoxmarket.com – darknet drug store
mostbet_gqEa
| #
мостбет мобильная версия скачать https://mostbet787.ru/ .
mostbet_kg_poka
| #
mostbet kg отзывы mostbet kg отзывы .
Alpha_Byte
| #
Ответы на частые вопросы о бизнесе: https://sinus.edu.pl/index.php/2022/12/21/booty-workout-sessions/
Slayer1831
| #
Что нельзя делать с финансами? https://taxhelps.ru
1win_depa
| #
1win com http://1win809.ru .
Rabyitabe
| #
dark web marketplaces https://github.com/darknetmarketlinks2025/darknetmarkets dark web sites
Pingrutty
| #
dark web drug marketplace https://github.com/darknetmarkets2025/darknetmarketlinks darknet markets links
FNDaviditabe
| #
darknet market list https://mydarkmarketlink.com/ – darknet market list
актуальное зеркало Старда
| #
Добро пожаловать в Starda Casino – мир невероятных выигрышей! Здесь вас ждут топовые развлечения от мировых провайдеров. https://starda-rush.buzz/ чтобы испытать удачу в лучших слотах!
Почему игроки выбирают Starda Casino?
Огромный выбор слотов от мировых производителей.
Выгодные акции с фриспинами и кешбэком.
Моментальные транзакции без задержек.
Оптимизированная мобильная версия для комфортной игры.
Круглосуточная служба поддержки отвечает в считанные минуты.
Создайте аккаунт и выиграйте по-крупному!
PhantomVoyager
| #
Памятка для работы с бизнесом: https://zelfrijdendetaxirotterdam.nl/uncategorized/verhuizen-naar-rotterdam-in-2024-dit-moet-je-weten/
Walker4918
| #
Примеры успеха https://concord-am.ru в финансах.
DonDonfaita
| #
onion dark website https://github.com/darkwebwebsites/darkwebwebsites darknet markets onion address
Willierex
| #
На нашем сайте вы откроете для себя новых девушек, которые ждут страстных встреч и интима. Каждая анкета — это целый мир, наполненный приключениями и интересами. Более 2500 реальных девушек готовы к общению и удовольствию. Найдите свою идеальную пару сегодня же https://individualki-sochi.life/
WilliamIRrutty
| #
darknet market lists https://darknetmarketsonion.shop/ – darknet markets
Shadow_Pioneer
| #
Использование на практике финансов: https://net-partners.ru
Kxyufaita
| #
dark market list https://cannahomemarket.link/ – darknet markets links
RedX3
| #
Мнение специалистов о бизнесе: https://keesinha.com/2020-um-ano-de-muitos-desafios/
Receipt Scanner App
| #
Overwhelmed by paper receipts? Say goodbye to financial disorganization with our cutting-edge financial tool!
Experience the convenience of AI-driven expense management.
Boost your tax deductions with clear overviews.
Unlock the potential of an app designed for maximum efficiency.
Take advantage of seamless https://play.google.com/store/apps/details?id=com.receiptscanner.app&hl=en_CA organization.
Mazrzxo
| #
Где приобрести диплом специалиста?
Получаемый диплом с приложением отвечает стандартам, никто не отличит его от оригинала. Не стоит откладывать личные мечты и цели на потом, реализуйте их с нашей компанией – отправляйте заявку на изготовление документа прямо сейчас! Диплом о среднем специальном образовании – не проблема! poluchidiplom.com/mozhno-li-kupit-attestat/
PhantomX1
| #
Экспертный уровень в финансах: https://bat-s.ru
vodka-fun.buzz
| #
Хотите испытать настоящий азарт? Тогда добро пожаловать в Vodka Casino – идеальное место для игры! Здесь вы найдете тысячи игровых автоматов от топовых брендов. https://vodka-fun.buzz/ и получить щедрые бонусы!
Что делает Vodka Casino особенным?
Широкий ассортимент игр с высоким RTP.
Выгодные акции на депозиты и ставки.
Гарантированные выигрыши на удобные платежные системы.
Приятный дизайн без зависаний и лагов.
Профессиональная служба помощи работают круглосуточно.
Зарегистрируйтесь в Vodka Casino и получите шанс на большие выигрыши!
Donaldlaf
| #
dark websites https://github.com/darkwebmarketslinks/darkwebmarkets darknet site
Hacker6835
| #
Тесты по финансам: https://express-sviaz.ru
MarkNOlaf
| #
dark websites https://drdarkfoxmarket.com – dark web sites
Iron_Walker
| #
Малоизвестные фишки бизнеса: http://www.bornfriedman.com/.
Toliknaf
| #
dark market onion https://github.com/darknetmarketslist/darknetmarketslist onion dark website
Code7530
| #
Сравнение лучших ресурсов по финансам: https://generalfactoring.ru
Eagle_Code
| #
С чего начать финансов? https://legko-zaim.ru
Pingrutty
| #
dark markets 2025 https://github.com/darknetmarkets2025/darknetmarketlinks darkmarkets
FNDaviditabe
| #
dark market list https://firstdarkmarket.com/ – dark web marketplaces
Rabyitabe
| #
darknet markets links https://github.com/darknetmarketlinks2025/darknetmarkets darknet markets onion address
Hacker2763
| #
Как сэкономить на бизнесе: https://sharktanktalesindia.com/fashion/most-overrate-kindness-greatest-be-oh-staking-laughter/.
WilliamIRrutty
| #
darknet marketplace https://kingdomdarkmarketonline.com/ – dark market onion
Jerrynoibe
| #
Glory Casino Sites
DonDonfaita
| #
darknet markets links https://github.com/darkwebwebsites/darkwebwebsites darknet links
DragonV9
| #
Как сэкономить на финансах: https://edinii-centr-kreditovaniya.ru
Kxyufaita
| #
darknet markets onion address https://cannahome-darkmarket-online.com/ – dark web sites
Red_Blade
| #
Рекомендую проверенный ресурс по финансам: https://cpp32.ru
Master3031
| #
Может кто знает разбирается в бизнесе? Куда зайти? https://kapilgupta100.com/multiple-sources-of-income/
MarkNOlaf
| #
dark web marketplaces https://kingdom-darkmarketplace.com – onion dark website
AlphaSentinel
| #
Нужны реальные кейсы по финансам. https://dos-96.ru
Donaldlaf
| #
darknet market lists https://github.com/darkwebmarketslinks/darkwebmarkets dark web market
Protocol9376
| #
Как https://giondragasuites.com/index.php/2023/08/24/halo-dunia/ преуспеть в бизнесе?
WolfX1
| #
Нестандартный подход к https://asir27.ru финансам.
Drenaj ychastkov v SPb_wrsi
| #
дренаж спб дренаж спб .
Pingrutty
| #
bitcoin dark web https://github.com/darknetmarkets2025/darknetmarketlinks darkmarket url
Drenaj ychastkov v SPb_qfsi
| #
выравнивание участка дренаж выравнивание участка дренаж .
Jerrynoibe
| #
Glory Casino – Gloryplay
StanleyLot
| #
Гарантируем безопасность обработки для детей и домашних животных https://dezcentr-spb.ru/
Toliknaf
| #
best darknet markets https://github.com/darknetmarketslist/darknetmarketslist darknet drug links
FNDaviditabe
| #
darkmarket link https://onion-dark-market.shop/ – dark market
WilliamIRrutty
| #
dark web drug marketplace https://idarknetmarket.com/ – dark market link
PhantomZ4
| #
https://asyrafreload.com/cara-transaksi-produk-tbo/ Практическое применение бизнеса.
CyberX5
| #
Как не сдаться https://sindikat-balance.ru при изучении финансов.
Iron_Warden
| #
Всем привет! Подскажите, https://tlk-kzn.ru где посмотреть информацию о финансах?
Rabyitabe
| #
darkmarket link https://github.com/darknetmarketlinks2025/darknetmarkets darkmarkets
Kxyufaita
| #
darknet markets onion address https://cannahome-darkmarket-online.com/ – darknet drug store
lkjhCype
| #
http://www.nonnagrishaeva.ru/press/pages/luchshie_filmu_na_temu_boevuh_iskusstv.html сериал мятеж 2020 смотреть онлайн бесплатно в хорошем качестве
Shadow375
| #
https://www.rizviaparty.com/volume-20142015/rizvia-party-album-2014/ Как избежать проблем с бизнесом?
Byte6439
| #
Что нового в https://finformula24.ru финансах?
DonDonfaita
| #
darknet market list https://github.com/darkwebwebsites/darkwebwebsites darknet drug store
MarkNOlaf
| #
dark market url https://dark-web-versus.com – darknet market list
Aurora играйте бесплатно
| #
Aurora Casino приглашает вас в мир высококлассных азартных игр и ярких эмоций. Каждый найдет здесь что-то по душе — от классических слотов до уникальных игр с реальными крупье. Участие в акциях и получение бонусов даст вам дополнительные шансы на выигрыш и сделает игру еще увлекательней.
Что делает https://auroracasino7.com/ лучшим выбором для игроков? Мы обеспечиваем полную безопасность ваших средств и конфиденциальность личных данных. Мы гарантируем прозрачные условия игры и мгновенные выплаты, чтобы вы могли наслаждаться выигрышами без задержек.
Когда стоит начать ваше путешествие в мире Aurora Casino? Регистрируйтесь прямо сейчас и получите доступ к множеству захватывающих игр и бонусов. Вот что вас ждет:
В Aurora Casino вас ждут регулярные бонусы и акции, которые увеличат ваши шансы на победу.
Мы гарантируем вашу безопасность и спокойствие, играя на нашей платформе.
Новинки и эксклюзивные игры.
С нами ваш путь к удаче будет интересным и прибыльным.
DarkVoyager
| #
Хочу понять в финансах. Что посоветуете? https://vashglbuh.ru
Gregoryfaf
| #
Kingmaker Kingmaker, Kingmaker Casino, Kingmaker greece Casino
LanceKew
| #
Prostat x?rc?ngin? qars? hans? qidalar istifad? edilm?lidir? https://x.com/Fariz418740/status/1902037337590108612
Pingrutty
| #
dark market onion https://github.com/darknetmarkets2025/darknetmarketlinks dark market list
ThunderWarden
| #
Как https://gistrade.ru вам этот ресурс о финансах?
FNDaviditabe
| #
dark markets https://onion-dark-market.shop/ – bitcoin dark web
WilliamIRrutty
| #
darknet markets url https://kingdommarketdarknet.com/ – darkmarkets
Toliknaf
| #
darkmarkets https://github.com/darknetmarketslist/darknetmarketslist darknet marketplace
ThunderSentinel
| #
5 главных ошибок при работе с финансами: https://ati-invest.ru
rox casino зеркало
| #
### Онлайн-казино: захватывающий мир азартных игр в интернете
Онлайн-казино стали популярным развлечением для миллионов игроков по всему миру. Они позволяют испытать удачу, насладиться атмосферой азартных игр и выиграть крупные суммы денег, не выходя из дома. Современные платформы предлагают огромный выбор игровых автоматов, рулетки, покера, блекджека и других игр, доступных в любое время суток.
#### Преимущества онлайн-казино
1. **Доступность** – играть можно с любого устройства: компьютера, смартфона или планшета. Все, что нужно, – это интернет-соединение.
2. **Большой выбор игр** – современные казино предлагают сотни, а иногда и тысячи слотов и карточных игр от ведущих разработчиков.
3. **Бонусы и акции** – новые игроки часто получают приветственные бонусы, фриспины и другие поощрения, которые увеличивают шансы на выигрыш.
4. **Безопасность** – лицензированные онлайн-казино используют передовые технологии шифрования данных, чтобы обеспечить защиту личной информации и финансовых транзакций.
5. **Игра в режиме реального времени** – благодаря живым дилерам пользователи могут наслаждаться атмосферой настоящего казино, находясь у себя дома.
#### Как выбрать надежное онлайн-казино?
Перед тем как начать игру, важно выбрать безопасную и честную платформу. Обратите внимание на:
– **Наличие лицензии** – надежные казино работают по лицензии официальных регуляторов (например, Malta Gaming Authority, Curacao eGaming и других).
– **Отзывы игроков** – изучите мнения других пользователей, чтобы узнать о репутации казино.
– **Выбор платежных методов** – удобные способы пополнения счета и вывода средств (банковские карты, электронные кошельки, криптовалюта).
– **Службу поддержки** – хороший игровой сервис всегда предоставляет круглосуточную поддержку клиентов.
#### Популярные игры в онлайн-казино
Игровые платформы предлагают разнообразие азартных развлечений:
– **Игровые автоматы (слоты)** – классические и современные слоты с яркой графикой, бонусными раундами и джекпотами.
– **Рулетка** – европейская, американская, французская версии игры с разными стратегиями ставок.
– **Покер** – техасский холдем, омаха, видео-покер и другие разновидности.
– **Блэкджек** – популярная карточная игра, где нужно набрать 21 очко и обыграть дилера.
– **Баккара** – динамичная игра, ставшая любимой среди профессионалов и новичков.
#### Советы для успешной игры
– Устанавливайте лимиты ставок, чтобы контролировать бюджет.
– Играйте только в лицензированных казино, чтобы избежать мошенничества.
– Используйте бонусные предложения, но внимательно читайте условия их отыгрыша.
– Развивайте стратегическое мышление в карточных играх, чтобы увеличить шансы на выигрыш.
– Помните, что азартные игры – это развлечение, а не способ заработка.
Онлайн-казино – это увлекательный мир азартных развлечений, который предлагает комфорт, удобство и возможность испытать удачу в любое время. Главное – подходить к игре ответственно, выбирать надежные площадки и получать удовольствие от процесса. Удачи за игровыми столами!
https://xteaser.ru/
Trefjwx
| #
Заказать документ о получении высшего образования вы сможете в нашей компании в Москве. diplomass.com/kupit-diplom-ulyanovsk-2
Kxyufaita
| #
darknet market lists https://cyberdarkmarkets.com/ – darknet drug store
Storm_Hacker
| #
https://czifron.ru Как преуспеть в финансах?
Rabyitabe
| #
dark markets 2025 https://github.com/darknetmarketlinks2025/darknetmarkets dark market url
JohnnyQualf
| #
Официальный веб-сайт казино Рояль предлагает располагающую обстановку для любителей казино. С его элегантным дизайном и высококачественным выбором игр, Рояль является оптимальным местом для тех, кто полагается качественное азартное развлечение. Исследуйте разнообразный выбор игровых автоматов и настольных игр на основном веб-сайте https://royalcasino-slots.com/.
Казино Рояль защищает высокий уровень клиентов и поддержку. Отзывчивая команда поддержки доступна круглосуточно, подготовлена откликнуться на различные вопросы и разрешить проблемы, обеспечивая беспрерывную игру и удовлетворение.
MarkNOlaf
| #
darkmarket list https://cannahomedarknetdrugstore.com/ – dark web market
CoreyLal
| #
учебный центр Переподготовка и повышение квалификации: инвестиции в профессиональный рост. В современном динамичном мире потребность в непрерывном обучении становится ключевым фактором успеха. Профессиональная переподготовка и курсы повышения квалификации – это эффективные инструменты для адаптации к меняющимся требованиям рынка труда и расширения профессиональных горизонтов. Если вы стремитесь к карьерному росту, желаете освоить новую специальность или углубить свои знания в уже имеющейся, курсы переподготовки предоставят вам необходимые компетенции. В ряде случаев возникает необходимость подтверждения квалификации. В таких ситуациях возможно купить удостоверение, соответствующее пройденному обучению. Особое внимание уделяется обучению в сферах, требующих повышенной безопасности. Обучение работе на высоте и безопасным работам на высоте, а также обучение по пожарной безопасности являются критически важными для обеспечения безопасности на рабочем месте. Для тех, кто ценит свое время и предпочитает гибкий график, курсы повышения квалификации дистанционно предоставляют возможность обучения в удобном формате. Обучение по профессиям охватывает широкий спектр специальностей, позволяя каждому найти подходящую программу.
LightX4
| #
Финансы для компаний: https://tandem585.ru
DonDonfaita
| #
darknet marketplace https://github.com/darkwebwebsites/darkwebwebsites darknet sites
NormanGaith
| #
Возможно ли получать тысячи манатов в месяц в соцсетях в Азербайджане?
https://pin.it/1OLQiA9c2
Walker7085
| #
Авторитетное мнение о финансах: https://chastnyj-investor.ru
Pingrutty
| #
dark web markets https://github.com/darknetmarkets2025/darknetmarketlinks darknet drugs
Terencesam
| #
Игровые автоматы лучшие Спины за регистрацию без депозита с выводом
FNDaviditabe
| #
darkmarket https://mydarkmarketlink.com/ – darknet sites
Lutherram
| #
Игровое заведение Вулкан – это увлекательный мир азарта, который завораживает с первого взгляда. Благодаря замечательному дизайну и понятному интерфейсу, пользователи быстро оказываются в этом игровом пространстве.
Среди преимуществ казино Вулкан игровые автоматы бесплатно стоит отметить широкий ассортимент игр, включая слоты и настольные развлечения, которые придутся по вкусу каждому игроку. Качество графики и анимационных эффектов делает игровой процесс дополнительно увлекательным и завораживающим.
Однако больше всего привлек внимание подход казино к безопасности игроков. Они используют новейшие технологии шифрования, чтобы обеспечить приватность личных и финансовых данных пользователей. Это делает казино Вулкан замечательным вариантом для онлайн-игры.
BruceHip
| #
голд казино
PhantomReaper
| #
Что лучше выбрать в финансах? https://lombardngs.ru
Glennbup
| #
уничтожение тараканов в москве
WilliamIRrutty
| #
darkmarket https://darknetmarketsonion.shop/ – darkmarket list
Donaldlaf
| #
dark web link https://github.com/darkwebmarketslinks/darkwebmarkets dark web marketplaces
DriverBaile
| #
Медицинский регистратор в поликлинике рекомендовал ознакомиться с важной медицинской информацией. Кремация организована с пониманием. Сотрудники работают профессионально.
Phantom_Nomad
| #
Ищу реальные кейсы по финансам. https://pfclub.ru
Arthurclevy
| #
Хотите получить bingo online 10 euros gratis начни игру без риска – Открой для себя захватывающие слоты, живое казино и эксклюзивные бонусы. Играй с комфортом, наслаждайся топовыми играми и выигрывай!
WilliamWAl
| #
Looking for top-notch slots to play? Abe Bet Casino offers endless variety online slots for all players!
At Abe Bet abe-casino.com/tr/games/demo/pragmatic_play_mustang_gold, you’ll find slot hits from top developers.
Register now to get generous bonuses.
Dive into an atmosphere of thrill with Abe Bet!
Kxyufaita
| #
darknet drug market https://kingdomdarknetdrugstore.com/ – dark web market
Toliknaf
| #
darknet websites https://github.com/darknetmarketslist/darknetmarketslist dark web market
Silver22
| #
С чего начать https://kpkmatkap.ru финансов?
mostbetuzbekistan
| #
Mostbet Uzbekistan mostbetuzbekistan-apk.com offers you profitable bets, top odds and instant payouts. Make sports predictions, play online casino and enjoy the excitement with registration bonuses!
Robertlox
| #
Ищете увлекательную платформу для азартных игр? Покердом — это лучший выбор для развлечений!
На https://kamuflyag-shop.ru/ вас ждут популярные слоты.
Простота интерфейса и программы лояльности делают игру еще интереснее!
Зарегистрируйтесь прямо сейчас и испытайте радость победы с Pokerdom!
MarkNOlaf
| #
best darknet markets https://drdarkfoxmarket.com – dark markets
Blue_Hunter
| #
Лучшие инструменты для работы с финансами: https://akcept-garant.ru
Rabyitabe
| #
darkmarket link https://github.com/darknetmarketlinks2025/darknetmarkets darkmarket 2025
EdwinFrima
| #
Ищете увлекательную платформу для азартных игр? Vulkan Russia — это идеальное место для развлечений!
На Vulkan Russia вас ждут захватывающие настольные игры.
Простота интерфейса и щедрые бонусы делают игру еще интереснее!
Зарегистрируйтесь прямо сейчас и испытайте радость победы с Вулкан Россия!
Pingrutty
| #
dark web sites https://github.com/darknetmarkets2025/darknetmarketlinks onion dark website
Daniellab
| #
Szukasz rzetelnego mechanika samochodowego w Poznaniu, który z pełnym zaangażowaniem zadba o Twoje auto i naprawi je na czas, zgodnie z obowiązującą gwarancją? Zaufaj zespołowi TAK Serwis: oferujemy kompleksowe usługi naprawy samochodów, w tym regularną wymianę części eksploatacyjnych – https://takserwis.pl/. Wybierając nasz warsztat samochodowy, postawisz na profesjonalną obsługę, wysokiej jakości części oraz szybką realizację prac.
Phantom_Slayer
| #
История внедрения финансов https://kpk-novydom.ru в компании.
Pinco Casino
| #
Джекпоты в онлайн-казино: реальные шансы на большой выигрыш
Современные азартные площадки предлагают захватывающие возможности для тех, кто ищет возможность ощутить удачу на своей стороне. Игроки могут не только насладиться разнообразием развлечений, но и надеяться на значительные выплаты. Но какова реальная вероятность получить желаемую сумму? В этой статье мы рассмотрим, на что стоит обратить внимание, чтобы повысить шансы на получение крупных выигрышей.
Статистические данные показывают, что суммарные выплаты в большинстве игр складываются из множества факторов, включая вероятность выпадения определенных сочетаний символов и правила самого автомата. Игрокам стоит обратить внимание на процент возврата игроку (RTP), который варьируется от 85% до 98%. Чем выше этот показатель, тем больше вероятность возврата вложенных средств.
Многие автоматы предлагают различные виды акций и бонусов. Оптимальное использование этих возможностей может значительно увеличить время игры и, следовательно, шансы на успех. Например, участвуя в турнирах, можно повысить как навыки игры, так и потенциал для получения крупных сумм. Также многие площадки предоставляют приветственные бонусы, которые увеличивают стартовый капитал и открывают новые горизонты для игроков, готовых рискнуть.
Как выбрать игровые автоматы
При выборе автоматов важно учитывать несколько ключевых факторов, чтобы повысить вероятность успеха. Во-первых, обратите внимание на высоту RTP (Return to Player). Этот показатель выражает, какую часть ставок игроки могут ожидать в виде выигрышей. Чем выше значение RTP, тем больше шансов возвращения средств. Идеальный вариант – автоматы с RTP выше 95%.
Кроме того, изучите волатильность игры. Высоковолатильные автоматы реже выдают выигрыши, но размер призов значительно больше. Низковолатильные аппараты, наоборот, часто приносят небольшие суммы. Выбор зависит от вашего стиля игры и готовности рисковать.
Не менее важным является разнообразие тематики и игровых функций. Автоматы с бонусными играми, фриспинами и дополнительными символами могут существенно увеличить увлекательность процесса и разнообразие возможностей для получения прибыли. Оцените доступные функции каждого автомата и выберите те, которые вам интересны.
Также стоит обратить внимание на репутацию разработчика. Известные компании часто гарантируют качество и честность игры. Читайте отзывы других игроков и выбирайте автоматы от проверенных брендов, чтобы избежать недобросовестных практик.
Не забывайте о демо-режиме. Многие платформы предлагают возможность пробовать автоматы без ставок, что позволяет ознакомиться с механикой и динамикой игры без финансовых рисков. Это особенно полезно для новичков, которые пока не уверены в своих предпочтениях.
Понимание вероятностей выигрыша
Чтобы оценить свои возможности, важно разобраться в механизмах формирования выплат, которые зависят от множества факторов. Каждый автомат имеет так называемый коэффициент возврата, который обозначает процент от всех ставок, возвращаемый игрокам. Стандартные значения колеблются от 85% до 98%, а это значит, что в долгосрочной перспективе игроки могут рассчитывать на разные уровни возврата.
Немаловажным аспектом является вероятность выпадения комбинаций. Для каждого конкретного устройства эта информация доступна в таблице выплат. Например, наличие редких символов, таких как специальные знаки или бонусные элементы, влияет на общую рентабельность. Чаще всего минимальные комбинации имеют более высокую частоту появления, тогда как крупные выплаты требуют больше времени и удачи.
Необходимо учитывать, что механика функции случайных чисел играет ключевую роль в формировании результата каждого спина. Поэтому даже при высоком проценте отдачи в краткосрочной перспективе возможны как выигрышные, так и проигрышные сессии. Тактика управления банкроллом поможет продлить игру и правильно распределить средства для получения максимального удовольствия.
Советы для более осознанного подхода включают проверку отзывов об играх, анализ актуальных статистических данных и использование демо-версий для знакомства с особенностями. Таким образом, смещение акцента на понимание механизмов увеличивает шансы на успех, несмотря на случайный характер событий.
https://905090.ru/
DonDonfaita
| #
darknet site https://github.com/darkwebwebsites/darkwebwebsites dark websites
FNDaviditabe
| #
darkmarket url https://mydarkmarketlink.com/ – darkmarket
Joshuamub
| #
Looking for best online casino games? MasalBet is the place to find favorite games from leading brands.
At https://masalbet-official.com/tr/, you’ll find exclusive promotions and a user-friendly interface.
Register now and get exclusive offers.
Play exciting games with MasalBet!
Guardian1235
| #
Пример из https://vp-finance.ru практики: Финансы.
WilliamIRrutty
| #
darknet site https://darknetmarketsonion.shop/ – dark market onion
mostbet_ueEr
| #
скачать mostbet на телефон http://mymoscow.forum24.ru/?1-2-0-00000718-000-0-0-1742357638/ .
1win_fcpi
| #
1win букмекер https://mymoscow.forum24.ru/?1-2-0-00000717-000-0-0-1742357431/ .
Michealhem
| #
https://stage.corich.jp/stage_main/234466
1win_hnon
| #
1 вин http://www.cah.forum24.ru/?1-19-0-00000732-000-0-0 .
Shadow_Blade
| #
Новые подходы в финансах: https://center-torgov.ru
Kxyufaita
| #
dark market list https://kingdomdarknetdrugstore.com/ – darknet links
Donaldlaf
| #
dark market link https://github.com/darkwebmarketslinks/darkwebmarkets darkmarket 2025
Blue573
| #
Эксклюзивное интервью с экспертом по финасам: https://gym-credit.ru
Toliknaf
| #
darknet markets onion https://github.com/darknetmarketslist/darknetmarketslist bitcoin dark web
MarkNOlaf
| #
darknet markets https://drdarkfoxmarket.com – darknet drug links
NightV9
| #
5 https://kapital-c.ru главных ошибок при работе с финансами.
DarkZ1
| #
Может кто знает ориентируется в финансах? Где искать? https://lombard-bis.ru
TrevorRat
| #
Магазин печей и каминов pech.pro широкий выбор дровяных, газовых и электрических моделей. Стильные решения для дома, дачи и бани. Быстрая доставка, установка и гарантия качества!
Pingrutty
| #
dark market url https://github.com/darknetmarkets2025/darknetmarketlinks darknet markets onion address
Hacker7694
| #
Как не сдаться при изучении финасов: https://fin-abc.ru
MichaelZoday
| #
whores ufc heroin
Rabyitabe
| #
darkmarkets https://github.com/darknetmarketlinks2025/darknetmarkets darknet sites
FNDaviditabe
| #
darknet drugs https://darkmarketdarkfox.com/ – darknet markets onion
WolfZ4
| #
Особенности реализации финансов: https://finiko-dv.ru
CoreyLal
| #
меховое ателье с Москве Мех как искусство: от норки до каракульчи, преображение и вдохновение Мир моды изменчив, но любовь к роскошному меху остается неизменной. Норка, каракульча – каждое полотно обладает своей неповторимой красотой и историей. Выбор шубы – это не просто приобретение теплой одежды, это инвестиция в элегантность и уверенность. В Москве и Санкт-Петербурге, в витринах бутиков и на распродажах, можно найти шубу своей мечты. Важно помнить, что скидки – это отличная возможность приобрести качественное изделие по выгодной цене. Но что делать, если в шкафу уже висит шуба, вышедшая из моды? Не спешите с ней прощаться! Авторский канал дизайнера предлагает вдохновляющие решения по перешиву и преображению меховых изделий. Дизайнер делится секретами, как вдохнуть новую жизнь в старую шубу, превратив ее в современный и стильный предмет гардероба. Кроме того, канал предлагает цитаты дня, мотивирующие на перемены и помогающие женщинам старше 60 лет почувствовать себя красивыми и уверенными в себе. Мех – это не просто материал, это способ выразить свою индивидуальность и подчеркнуть свой неповторимый стиль.
DonaldWheri
| #
каталог nova маркетплейс
WilliamIRrutty
| #
darknet markets onion https://kingdomdarkmarketonline.com/ – dark web marketplaces
Master4685
| #
Читал прогнозы, что Финансы будет главным трендом. Возражения? https://finansovoereshenie.ru
DonDonfaita
| #
darkmarket https://github.com/darkwebwebsites/darkwebwebsites darknet markets onion
Binesahaw
| #
Join the adventure in Gameworld Barbarossa today. Begin fete medievale saint valery sur somme your quest for riches with immersive gameplay, legendary characters, and massive payouts designed to captivate every treasure-hunting player.
jozz casino
| #
Можно ли обыграть онлайн-казино? Разбираем популярные мифы
Виртуальные азартные заведения давно стали объектом обсуждения, и среди игроков активно циркулирует множество заблуждений. Некоторые утверждения представляют собой простую дезинформацию, в то время как другие основаны на честном желании поделиться опытом. Тем не менее, важно разобраться в фактах и вычленить истинное из потока домыслов. Актуальные статистические данные показывают, что лишь небольшой процент участников действительно достигает значительных успехов.
Некоторые убеждены, что с помощью определённых стратегий можно гарантировать выигрыш. Однако, многие рекомендации, такие как использование систем ставок или выбор определённых игр, не обеспечивают ожидаемой прибыли. Результаты в большинстве случаев зависят от случайности. Математические модели подсказывают, что долговременное преимущество находится на стороне заведения, и лишь временные успехи отдельных игроков не меняют общую картину.
В то же время существуют игровые механики, которые могут помочь улучшить баланс игровых сессий. Изучение правил и дисциплина при управлении капиталом становятся залогом для осознанного подхода к развлечению. Важно обратить внимание на использование бонусов и акций, которые могут несколько увеличить шансы, но и здесь следует помнить о рисках. Информированность – ключ к уверенности на пути к развлечение в цифровом пространстве азартных игр.
Скептические взгляды на ставки
Существует распространенное мнение, что применение определённых систем ставок всегда приводит к успеху. Многие азартные участники полагают, что существует универсальная стратегия, способная гарантировать прибыль при игре в азартные развлекательства. Важно понимать, что такие подходы обычно основываются на неверных предположениях о механике игр. Например, монетная система требует увеличения ставок после каждой проигрышной игры, однако она не меняет шансы на выигрыш.
Другой миф заключается в том, что последовательность выигрышей или проигрышей может указывать на будущие результаты игры. Распространенное заблуждение о “горячих” или “холодных” числах в игре в рулетку является иллюзией. Фактически, каждая новая игра независима от предыдущей, и результаты не имеют взаимосвязи друг с другом.
Некоторые игроки убеждены, что использование сложных математических формул и стратегии может значительно повысить вероятность выигрыша. Однако, несмотря на высокую математическую теорию, игры, основанные на случайных числах, не поддаются контролю, и такие методы не обеспечивают надёжной защиты от потерь.
Важно учитывать, что азартные игры – это, прежде всего, развлечение. Разумный подход заключается в установлении чётких лимитов на ставки и понимании того, что никакая система не гарантирует успех. Продумывая стратегию, полезно опираться на финансовые ограничения и личные предпочтения, а не на иллюзии о системе ставок.
Реальные стратегии выигрыша
Существуют различные подходы к игре, зависящие от особенностей конкретных азартных развлечений. Один из таких методов – применение ставок по системе Мартингейл. Она заключается в удвоении ставки после каждого проигрыша. Это позволяет в случае выигрыша вернуть все предыдущие потери и получить небольшую прибыль. Однако такой метод требует достаточного банковского ресурса и мудрого управления финансами.
Другой метод – использование стратегии минимизации рисков. Например, в рулетке рекомендуется делать ставки на цвет или четность, так как они предлагают высокий шанс на выигрыш (приблизительно 48%). Данный подход уменьшает вероятность крупных потерь и создает возможность для длительной игры с разумным банкроллом.
Следующий вариант – анализ в игровых аппаратах. Многие игроки игнорируют фактические шансы конкретных слотов. Исследование уровня возврата к игроку (RTP) помогает выбирать автоматы с более высоким процентом возврата, что увеличивает вероятность успеха в долгосрочной перспективе.
В блэкджеке нарастает популярность стратегий, базирующихся на подсчете карт. Игроки отслеживают, какие карты уже были розданы, чтобы оценить вероятность появления высоких карто. Хотя этот подход запрещен во многих заведениях, его использование все же может дать значительное преимущество.
Наконец, важным аспектом является дисциплина. Установив заранее предел для выигрышей и проигрышей, можно избежать эмоциональных решений, которые часто приводят к независимым потерям. Фиксация результатов каждой сессии и анализ их позволит выявить свои сильные и слабые стороны, что приведет к улучшению стратегии игры.
https://ss-net.ru/
Reaper5123
| #
Где смотреть https://stopkredit-vladivostok.ru актуальные данные о финансах?
Kxyufaita
| #
darknet sites https://darkfoxmarketplace.com/ – darknet links
https://wiki.flashcorponline.com/index.php/Почему_Зеркала_Слотозал_Промокод_Так_Ðезамнимы_ДлÑ_Ð’Ñех_К
| #
https://wiki.flashcorponline.com/index.php/Почему_Зеркала_Слотозал_Промокод_Так_Ðезамнимы_ДлÑ_Ð’Ñех_Клиентов
A Brief History of Elemental Analysis » Artemis Analytical
1win_sqpi
| #
1win.pro http://mymoscow.forum24.ru/?1-2-0-00000717-000-0-0-1742357431 .
mostbet_nwEr
| #
mostbet.kg mostbet.kg .
EdwardLable
| #
go to my blog acheter des billets contrefaits
Donaldlaf
| #
darknet drug links https://github.com/darkwebmarketslinks/darkwebmarkets darknet market lists
1win_hjon
| #
1win.kg http://www.cah.forum24.ru/?1-19-0-00000732-000-0-0 .
MarkNOlaf
| #
darknet drug links https://drdarkfoxmarket.com – dark web marketplaces
EagleHunter
| #
Исследование финансов: https://rostpension-partner.ru
RedWarden
| #
Пошаговая инструкция по финансам: https://kpk-ruscapital.ru
Pingrutty
| #
dark web marketplaces https://github.com/darknetmarkets2025/darknetmarketlinks darkmarket 2025
Toliknaf
| #
darkmarket list https://github.com/darknetmarketslist/darknetmarketslist dark markets
FNDaviditabe
| #
dark web marketplaces https://darkmarketdarkfox.com/ – best darknet markets
VortexGuardian
| #
Профессионалы рекомендуют эти ресурсы по финансам: https://prostolombard.ru
WilliamIRrutty
| #
darkmarket 2025 https://idarknetmarket.com/ – darknet market list
Eagle801
| #
Где тренироваться финансам? https://finansist-spb.ru.
Rabyitabe
| #
darknet market https://github.com/darknetmarketlinks2025/darknetmarkets dark web market
Kxyufaita
| #
best darknet markets https://kingdomdarknetdrugstore.com/ – dark web link
DonDonfaita
| #
dark web markets https://github.com/darkwebwebsites/darkwebwebsites dark web market list
DragonWarden
| #
https://onegokredit.ru Современные методы по финансам.
Sazrkzv
| #
Мы изготавливаем дипломы любых профессий по приятным ценам. Стоимость может зависеть от выбранной специальности, года выпуска и образовательного учреждения. Всегда стараемся поддерживать для покупателей адекватную ценовую политику. Важно, чтобы документы были доступными для большого количества граждан. купить диплом университета в ростове на дону
Voyager5169
| #
Смешные случаи в финансах: https://ravoskhod.ru.
MarkNOlaf
| #
dark market https://kingdomdarkmarketplace.com – dark web market
Pingrutty
| #
darknet links https://github.com/darknetmarkets2025/darknetmarketlinks dark web link
Cazrfwk
| #
Здравствуйте!
Мы изготавливаем дипломы любых профессий по разумным ценам. Стоимость будет зависеть от выбранной специальности, года получения и образовательного учреждения: diplomans.com/
Donaldlaf
| #
darkmarket list https://github.com/darkwebmarketslinks/darkwebmarkets darknet drugs
Cazrtax
| #
Приобрести диплом ВУЗа по невысокой стоимости возможно, обратившись к надежной специализированной компании. Мы готовы предложить документы престижных ВУЗов, расположенных в любом регионе РФ. diplomc-v-ufe.ru/kupit-diplom-texnikuma-moskva
RedHacker
| #
Какие https://galactic-pro.ru изменения в финансах?
FNDaviditabe
| #
darkmarket 2025 https://kingdom-marketplace.com/ – dark web market urls
PhantomPioneer
| #
Какие платформы использовать для финансов? https://pegascapital.ru
WilliamIRrutty
| #
tor drug market https://kingdomdarkmarketonline.com/ – dark web market urls
ShadowZ9
| #
Смешные случаи в финансах: https://lombardchernozemie.ru
Kxyufaita
| #
dark web sites https://cannahomemarket.link/ – dark market
DragonZ5
| #
Приветствую. Подскажите, где найти информацию о финансах? https://vis-invest.ru
Diplomi_pjKt
| #
Приобрести диплом ВУЗа!
Мы готовы предложить документы институтов, расположенных на территории всей России.
diplomj-irkutsk.ru/kupit-diplom-o-srednem-obrazovanii-11-klassov-4
BlueSentinel
| #
Ошибки новичков в финансах: https://kpkdoverierm.ru.
MarkNOlaf
| #
dark market onion https://dark-web-versus.com – dark market list
Stephenavelo
| #
септики для дома купить спб купить септик для частного дома
FNDaviditabe
| #
darknet drugs https://firstdarkmarket.com/ – darknet market links
DarkByte
| #
С чего начать финансов? https://zmd-region.ru
WilliamIRrutty
| #
darknet markets url https://idarknetmarket.com/ – darknet drug market
Ghost_Guardian
| #
Секретные методы https://buildrich.ru финансов.
Thomasprise
| #
Как научить ребёнка контролировать гнев? Покажите, что можно злиться, но при этом не обижать других. Например, предложите ему “поругаться” с подушкой или нарисовать свои чувства. Объясните, что даже взрослые иногда злятся, но умеют с этим справляться.
play fortuna зеркало
| #
Какие бонусы самые выгодные в 2025 году?
В последнее время наблюдается заметное изменение подходов к программам поощрения клиентов. В условиях растущей конкуренции компании стремятся предложить более таргетированные и привлекательные предложения, позволяя пользователям выбирать те, которые действительно отвечают их потребностям и образу жизни. В этом контексте особенно интересны предложения, которые показывают реальную ценность на фоне других опций.
Один из наиболее многообещающих трендов – это программы лояльности с гибкими условиями. Потребители теперь могут накапливать баллы и располагать ими по своему усмотрению, например, использовать их не только на покупки, но и для получения услуг, таких как подписка на стриминг или аренда автомобилей. Такой подход позволяет удовлетворить разнообразные потребности клиентов и значительно повышает степень вовлеченности.
Другим примером могут служить кэшбэк-программы, которые, как показывают исследования, становятся все более популярными среди пользователей. Совершая повседневные покупки, клиенты будут возвращать до 10% от суммы, что существенно экономит средства и повышает общую привлекательность продуктов и услуг. При этом важно обращать внимание на условия: многие компании предлагают кэшбэк только при определенных условиях или через конкурирующие платформы.
В сочетании с современными технологиями, такими как мобильные приложения и персонализированные предложения, эти новые форматы делают программу поощрения более доступной и понятной, что создает благоприятную атмосферу для пользователей. На 2025 год, выбор подходящей опции будет зависеть от индивидуальных предпочтений, активности и конкретных жизненных ситуаций. Сравнение нескольких вариантов и анализ их достоинств помогут сделать правильный выбор.
Преимущества для инвесторов
Инвесторы в 2025 году могут воспользоваться рядом предложений, способствующих увеличению доходности. Открытые инвестиционные платформы часто предлагают акции на комиссии. Это позволяет минимизировать затраты при входе в новые позиции или при работе с портфелем.
Некоторые финансовые компании вводят программы возвращения части средств. Такие инициативы могут быть применимы к инвестициям в определённые финансовые инструменты. Например, пользователи могут получить кэшбэк за торговлю акциями или ETF, что значительно увеличивает общий доход.
Но не только возврат средств становится привлекательным. Для тех, кто активно использует кредитные средства, важно обратить внимание на предложения с низкими процентными ставками. Особенно актуально это для заимствований на торговые сделки. Низкие ставки способны повысить рентабельность проектов за счёт сниженных издержек.
Не стоит упускать из виду и возможности, связанные с налоговыми льготами. Многие страны предлагают специальные условия для инвесторов. Участие в таких инициативах может привести к значительной экономии, особенно если инвестирование осуществляется в долгосрочной перспективе.
Также обратите внимание на образовательные программы от брокеров. Качественное обучение может открыть новые стратегии и подходы к инвестированию. Например, вебинары и курсы по техническому анализу или инвестиционным психологиям могут значительно улучшить результаты ваших торгов.
В 2025 году бизнес-модели многих компаний будут адаптироваться к новым условиям рынка. Это создаёт уникальные возможности для инвесторов, готовых исследовать и пробовать новые подходы. Ищите предложения, которые не только улучшают ваш финансовый результат, но и увеличивают ваши знания, что, в итоге, приведёт к более взвешенным решениям в области инвестиций.
Промоакции в ритейле
В 2025 году многие компании в ритейле применяют разнообразные промоакции, чтобы привлечь покупателей. Скидки на 10-50% становятся стандартом, при этом нередко встречаются эксклюзивные предложения на ограниченные сроки. Интересный пример – акции на популярные товары, которые напрямую зависят от времени года, праздников или особых событий.
Кэшбек-системы также продолжают набирать популярность. Такая схема позволяет клиентам возвращать часть потраченных средств, что стимулирует повторные покупки. Важно выбрать ритейлеров, которые предлагают кэшбек на высокие суммы или по ценным категориям товаров.
Система лояльности играет значимую роль в построении долгосрочных отношений с клиентами. Программы, в которых за каждую покупку начисляются баллы, которые затем можно обменять на скидки, подарки или специальные предложения, всегда привлекают внимание. Рекомендуется делать акцент на те программы, которые предлагают максимальное количество точек и гибкие условия обмена.
Сезонные акции представляют собой отличный способ стимулировать спрос. Например, праздничные распродажи или заранее анонсированные дни скидок позволяют ритейлерам не только увеличить оборот, но и разгрузить запасы товара. Такие подходы часто итеративны и базируются на потребительских предпочтениях и данных о продажах.
Следует также обратить внимание на акционные наборы. Упаковка нескольких товаров по специальной цене позволяет не только увеличить средний чек, но и привлечь внимание к менее известному ассортименту. Перед запуском подобных акций полезно провести исследование мнений потребителей, чтобы выявить сочетания товаров, которые будут интересны целевой аудитории.
https://pizzanyam.ru/
Kxyufaita
| #
dark web market urls https://cannahomemarket.link/ – dark websites
Dragon451
| #
Что https://czifronpay.ru лучше выбрать в финансах?
hMustafaVep
| #
Какой красивый оттенок! Заказала букет маме.
Доставка цветов Томск
GhostV3
| #
Преодоление трудностей при изучении https://tsb-broker.ru финансов.
Red420
| #
Пошаговая инструкция https://ik-rodina.ru по финансам.
JamesFeque
| #
В нашем списке лучшие девушки города Адлер — это топ реальных индивидуалок, каждая из которых стремится оставить незабываемые воспоминания. Погрузитесь в атмосферу нежности и страсти, открывая новые возможности для общения и отношений, которые помогут вам стать ближе к своим мечтам https://night24-adler.com/
Thomasseato
| #
Предоставим легальный адрес и полный пакет документов для регистрации: временная регистрация москва
FNDaviditabe
| #
dark market url https://mydarkmarketlink.com/ – dark market 2025
DennisSen
| #
porn esports whores
ThomasVep
| #
Цветы очень свежие и ароматные, жена была в восторге!
заказать цветы с доставкой в томске
BMW_ppKt
| #
Новый BMW X6: стиль и мощь, модельный ряд.
Превосходство BMW X6 на дороге, всегда.
Откройте для себя.
Уникальный дизайн BMW X6, высокой техники.
Как BMW X6 меняет правила игры, откройте.
Идеальный выбор – BMW X6, инвестирование.
Потрясающая отделка и материалы в BMW X6, создают.
Ваш надежный спутник – BMW X6, предоставляет.
Почему стоит выбрать BMW X6?, в нашем обзоре.
Динамичный BMW X6 – для активной жизни, каждого.
Как BMW X6 заботится о вашей безопасности, в приоритете.
Выбор BMW X6: ваши преимущества, открыл.
Технологический прогресс BMW X6, формируют.
Как будет ощущаться поездка на BMW X6, особенности.
Что дает вам BMW X6?, в нашем анализе.
Выразительный дизайн BMW X6, подчеркнет ваш статус.
Как BMW X6 выглядит на фоне конкурентов, в нашем сравнении.
Что говорят владельцы о BMW X6?, в нашей статье.
Современные системы безопасности BMW X6, позаботятся о вас.
Заключение: стоит ли покупать BMW X6?, обобщаем мнение.
bmw 2 bmw 2 .
WilliamIRrutty
| #
darknet markets onion address https://kingdommarketdarknet.com/ – dark market
RichardVarse
| #
whores esports heroin
Kxyufaita
| #
dark market 2025 https://kingdomdarknetdrugstore.com/ – dark markets
EagleZ4
| #
Мифы и правда о финансах: https://mqncwltd.ru
Steel388
| #
Смешные случаи в финансах: https://investd.ru.
1win_jfki
| #
1win com http://www.1win705.ru .
mostbet_pjml
| #
mostbet http://mostbet780.ru/ .
mostbet_kg_tber
| #
мотбет http://www.mostbet781.ru .
Byte1034
| #
Лучшие инструменты для работы с финансами: https://pik-24.ru
Modelnyy_lmOt
| #
Модельный ряд BMW: откройте для себя новые возможности, которые порадуют.
Узнайте о лучших моделях BMW, которые впечатляют.
Откройте для себя новейшие модели BMW, спортивные машины.
Каждый найдет свою идеальную BMW, впечатляюще широк.
Погрузитесь в мир премиальных автомобилей BMW, которые создают.
Что отличает BMW от других брендов, обнаружьте.
Каждая модель BMW — это шедевр, созданный для.
Лучшие автомобили в модельном ряду BMW, которые заинтересуют.
Как BMW отвечает на вызовы времени, проверьте.
Новый взгляд на автомобили BMW, подходящие для городской жизни.
BMW — это больше, чем просто автомобиль, это символ статуса.
Исключительное качество: выбор BMW, который вдохновляет.
Переосмысленный комфорт и элегантность BMW, поклонников.
Почему BMW — это ваш идеальный выбор, от комфорта до инноваций.
Давайте исследовать великолепие модельного ряда BMW, с передовыми технологиями.
Наслаждайтесь разнообразием автомобилей BMW, для любых приключений.
Модельный ряд BMW: сочетание стиля и технических решений, для тех, кто ищет лучшее.
Откройте для себя свою следующую BMW, с надежностью и безопасностью.
Каждый автомобиль BMW — это возможность, для любого владельца.
x3 bmw https://model-series-bmw.biz.ua/ .
FNDaviditabe
| #
dark web market list https://mydarkmarketlink.com/ – dark web sites
BryanCar
| #
вулкан казино зеркало
WilliamIRrutty
| #
darkmarket https://kingdommarketdarknet.com/ – dark websites
DragonReaper
| #
Лучшие инструменты для работы с https://makapital.ru финансами.
Thunder375
| #
Может кто знает разбирается в https://strahovkatut21.ru финансах? Куда зайти?
Kxyufaita
| #
darknet links https://cannahomemarket.link/ – darknet drug market
JamesFeque
| #
У нас представлены лучшие девушки Адлера, ставшие частью топа реальных индивидуалок. Каждая девушка подарит вам уникальную возможность найти понимание и близость. Погрузитесь в мир настоящего счастья и нежных чувств, о которых вы так долго мечтали: секс адлер
Sazrvam
| #
Приобрести диплом университета !
Покупка диплома университета РФ в нашей компании – надежный процесс, поскольку документ заносится в реестр. При этом печать осуществляется на специальных бланках ГОЗНАКа. Приобрести диплом любого ВУЗа arenadiplom24.online/vuzy/fgou-vpo-ngpu
IronWalker
| #
Приветствую. Может кто в курсе, где найти информацию о финансах? https://titanconsultgroup.ru
Eagle_Voyager
| #
Забавные https://avanschita.ru моменты в финансах.
Eddiehem
| #
whores esports terrorist attack
RafaelBax
| #
heroin esports porn
FNDaviditabe
| #
dark market 2025 https://kingdom-marketplace.com/ – darknet links
WilliamIRrutty
| #
darknet drug market https://idarknetmarket.com/ – dark web market list
1win_rnki
| #
1win cazino https://www.1win705.ru .
Blue_Guardian
| #
Полное руководство по https://vam-zaim56.ru финансам от А до Я.
1xslots казино
| #
Разбираем популярные системы ставок в блекджеке
Карточная игра, где мастерство и удача идут рука обруку, привлекает внимание азартных игроков по всему миру. Здесь выбор стратегии может существенно повлиять на итоговый результат. Наблюдая за мастерами, можно заметить, что многие из них опираются на разнообразные подходы, которые помогают управлять банкроллом и максимизировать шансы на выигрыш.
Одним из наиболее исследуемых методов является система, основанная на постепенном увеличении размера ставки после проигрыша. Эта тактика, известная как “мартингейл”, позволяет игрокам рассчитывать на быстрое возмещение потерь, однако риск потери значительной суммы при затяжной серии неудач остается высоким. Использование такой стратегии требует особого контроля за лимитами и объемом доступных средств.
Среди других методов выделяется концепция прогрессивного увеличения ставок после выигрыша. Она нацелена на извлечение выгоды в периоды удачи, однако и она не лишена подводных камней. Важно учитывать, что игровые сессии могут быть непредсказуемыми, и после успешного результата может последовать ряд поражений.
Разработка индивидуальной стратегии, опирающейся на собственные наблюдения и азартные привычки, может помочь достичь поставленных целей. Главное – это адаптивность и критическое мышление, которые позволят в нужный момент пересмотреть подход и минимизировать риски при игре с картами.
Система Мартингейла
Мартингейл основывается на принципе удвоения ставки после проигрыша. Идея проста: если игрок теряет, он увеличивает ставку, чтобы при следующей победе покрыть все предыдущие убытки и заработать первоначальную прибыль. Это требует наличия достаточного банкролла и устойчивости к рискам.
Стратегия предполагает, что игрок действует по следующему алгоритму: начать с небольшой суммы, затем удвоить ставку после каждого проигрыша. Например, если первая ставка составила 10 единиц и она проиграна, следующая ставка будет 20, затем 40, и так до тех пор, пока не произойдет выигрыш или банкролл не истощится.
Важно учитывать, что при использовании Мартингейла у игроков могут возникнуть проблемы на длинной дистанции. Поскольку ставки могут быстро увеличиваться, существует риск достижения лимитов стола или полного исчерпания средств. Поэтому установка максимального ограничения для ставки является разумным решением.
Существуют нюансы, которые стоит учитывать: играя на малом лимите, игрок может не столкнуться с серьезными проблемами, но при высоких ставках в ситуациях с длительными проигрышами вероятность банкротства значительно возрастает. Перед началом игры важно определить четкий план и придерживаться его.
Некоторые эксперты рекомендуют сочетать Мартингейл с другими стратегиями, такими как фиксированные ставки или другие системы управления банкроллом, чтобы сгладить риски и повысить шансы на победу. Также полезно периодически пересматривать свою стратегию, адаптируя её к текущей ситуации за столом.
Система Д’Alembert
Система Д’Alembert основана на принципе балансировки потерь и выигрышей. Основной метод заключается в увеличении ставки на одну единицу при проигрышах и уменьшении ее на одну единицу при выигрыше. Такой подход минимизирует риски при последовательных проигрышах и позволяет восстанавливать потери.
Для начала игры определите первоначальный размер вашей ставки. Лучше всего использовать небольшую сумму, чтобы снизить уровень риска. Многие игроки начали с такой ставки, которая составляет всего 1% от своего банкролла. Это позволит вам более гибко реагировать на ситуацию за столом.
Например, если начальная ставка составляет 10 рублей, при проигрыше вы ставите 11 рублей, затем 12 рублей и так далее. При выигрыше возвращаетесь к предыдущей ставке. Такой алгоритм помогает сохранять банкролл и не дает ему стремительно уменьшаться при неблагоприятной серии.
Несмотря на свою простоту, Д’Alembert не является безрисковым. Зависимость от последовательности выигрышей и проигрышей может привести к значительным потерям, если не учесть верхний предел ставок. Устанавливая лимит на максимальную ставку, вы защитите себя от крупных убытков.
Как рекомендация, старайтесь следить за своей игрой и отмечать результаты, чтобы оценить эффективность данной тактики для вашей конкретной ситуации. Пристальное внимание к своему банкроллу и контроль над эмоциями могут существенно повысить шансы на успех в долгосрочной перспективе.
https://elainexpress.ru/
mostbet_ptml
| #
мосбет казино http://www.mostbet780.ru .
Kxyufaita
| #
darknet markets https://darkfoxmarketplace.com/ – darknet market list
mostbet_kg_eser
| #
mosbet https://www.mostbet781.ru .
binance register
| #
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Blade2343
| #
Видел прогнозы, что Финансы https://at-agency.ru будет главным трендом. Возражения?
OmegaStriker
| #
Вебинар о финансах: https://prostzaim.ru
Howardrat
| #
Kraken darknet market – Кракен вход через тор, Даркнет вход
FNDaviditabe
| #
dark market 2025 https://firstdarkmarket.com/ – dark web drug marketplace
SteelZ1
| #
Технические нюансы https://kmv-lombard.ru финансов.
BryanCar
| #
казино онлайн играть
WilliamIRrutty
| #
dark web market list https://idarknetmarket.com/ – darknet markets links
Guardian9690
| #
Лучшие материалы по финансам: https://metiz-krovly.ru
Thomasseato
| #
Мы поможем оформить регистрацию по месту пребывания без очередей и бюрократии: сколько стоит временная регистрация в спб на 1 год для граждан рф
Kxyufaita
| #
darknet market list https://darkfoxmarketplace.com/ – darknet markets links
SteelHunter
| #
Топовый ресурс по теме финансов: https://kreditchelny.ru
GhostX1
| #
Предлагаю подискутировать о финансах: https://ber-kar.ru.
Warden5606
| #
https://vp-ipoteka.ru Рекомендую качественный ресурс по финансам.
FNDaviditabe
| #
dark web drug marketplace https://firstdarkmarket.com/ – bitcoin dark web
WilliamIRrutty
| #
dark web market list https://kingdommarketdarknet.com/ – darknet markets 2025
Iron_Overlord
| #
Особенности реализации финансов: https://buhgalterskaya-pomosh.ru
Kxyufaita
| #
tor drug market https://cannahomemarket.link/ – dark websites
lasuzFus
| #
Все о новом фильме говорят, но если вы его не хотите смотреть, тогда на сайте fankino.ru прочтите краткое описание, чтобы в курсе быть. На сайте найдете много нового и интересного для себя. Мы собрали для вас смс поздравления, красивые картинки, народные приметы. Ищете адвокатъ ардашевъ тайна персидского обоза сериал с 2019 г? Fankino.ru – портал с понятным и простым интерфейсом. Тут размещаем актуальные ответы и вопросы. Вы узнаете, где можно покупаться на Крещение. Найдете на умение и способность любить тест. Также вас ждут увлекательные головоломки. Уверены, что вам понравится у нас!
Cyber_Reaper
| #
Профессионалы рекомендуют эти ресурсы по финансам: https://metiz-saiding.ru
CyberSpark
| #
Преодоление трудностей при изучении https://finanskusa.ru финансов.
FNDaviditabe
| #
darknet drug links https://firstdarkmarket.com/ – dark web link
WilliamIRrutty
| #
darknet market https://darknetmarketsonion.shop/ – dark market 2025
Byte9796
| #
Полезные сервисы для работы с финансами: https://kpk-ugra.ru
Storm639
| #
Как работает механизм финансов? https://ygroup-sochi.ru
Kxyufaita
| #
darknet marketplace https://cannahomemarket.link/ – darknet market
Sazrnay
| #
Мы готовы предложить дипломы любой профессии по доступным тарифам. Основные преимущества заказа документов в нашем сервисе
Вы покупаете диплом в надежной и проверенной временем компании. Такое решение позволит вам сэкономить не только много средств, но и время.
На этом плюсы не заканчиваются, их куда больше:
• Документы печатаются на настоящих бланках с мокрыми печатями и подписями;
• Дипломы всех ВУЗов и ССУЗов России;
• Цена значительно ниже той, которую пришлось бы заплатить за обучение в университете;
• Доставка в любые регионы Российской Федерации.
Заказать диплом о высшем образовании– http://infinityaccess.org/read-blog/385_kupit-attestat-8-klass.html/ – infinityaccess.org/read-blog/385_kupit-attestat-8-klass.html
Nomad9891
| #
Всем привет! Может кто в курсе, где посмотреть информацию о финансах? https://pbmotsenka.ru
Eanrxao
| #
Для быстрого продвижения по карьере требуется наличие официального диплома института. Выгодно приобрести диплом ВУЗа у надежной организации: diplomh-40.ru/kupit-diplom-v-ekaterinburge-15/
Byte8651
| #
Видеоуроки по финансам: https://lombard-goldenstone.ru.
FNDaviditabe
| #
dark web market https://firstdarkmarket.com/ – dark web markets
Voyager6277
| #
Как реализовали финансов в компании: https://winfinsochi.ru
WilliamIRrutty
| #
dark web market urls https://darknetmarketsonion.shop/ – darknet markets url
Kxyufaita
| #
darkmarket 2025 https://cannahomemarket.link/ – darknet market
SilverCode
| #
Подкаст о финансах: https://financegroup24.ru
Red160
| #
Авторитетное мнение о финансах: https://lombard2x2.ru
Davidcal
| #
https://ruspress24.ru/
BluePioneer
| #
Примеры успеха https://vsekredity-tmn.ru в финансах.
Bryanonefs
| #
Some are medicines that help people when doctors prescribe.
can you get lisinopril
A touchstone of international pharmacy standards.
FNDaviditabe
| #
dark web market https://darkmarketdarkfox.com/ – darkmarket list
Silver_Overlord
| #
Предлагаю подискутировать о финансах: https://yard-capital.ru
WilliamIRrutty
| #
dark market 2025 https://asap-market-onion.com/ – darkmarket
StormCode
| #
Как выбрать лучшее решение для финансов: https://samtrader.ru
Kxyufaita
| #
dark web markets https://cannahome-darkmarket-online.com/ – tor drug market
Davidcal
| #
Северо-Запад РФ
StormV4
| #
Как сэкономить на финансах: https://kredit-ivanovo.ru
JeffreyVet
| #
Bukmacher oraz kasyno internetowe Mostbet to jeden z najdłużej działających serwisów hazardowych w sieci z ofertą dla Polaków, gdyż został założony jeszcze w 2009 roku. Platforma może pochwalić się posiadaniem aktywnej licencji od Curacao, która gwarantuje bezpieczeństwo. Gracze będą mogli spotkać tutaj ponad 3000 zróżnicowanych gier hazardowych, które zostały podzielone na automaty, gry stołowe oraz gry na żywo. Nie zabraknie też interesującego dodatku w formie różnorodnych gier natychmiastowych, z czego najbardziej wyróżniają się gry natychmiastowe. Platforma Mostbet Casino udostępnia jednocześnie wiele zróżnicowanych tytułów od najbardziej popularnych dostawców oprogramowania, wśród których znajdują się takie uznane marki jak na przykład Play’n GO, Pragmatic Play, Evolution, Microgaming czy NetEnt. Do tego dochodzą różnorodne bonusy, promocje czy turnieje.
Light_Slayer
| #
По данным аналитиков, лучший источник о финансах – https://antey-anapa.ru
Miguelbok
| #
казино
FNDaviditabe
| #
darknet drugs https://firstdarkmarket.com/ – darknet markets onion
WilliamIRrutty
| #
darkmarket list https://darknetmarketsonion.shop/ – tor drug market
Spark7634
| #
Новые подходы в финансах: https://balykovgroup.ru
Uazroer
| #
Привет!
Для многих людей, купить диплом университета – это острая потребность, удачный шанс получить хорошую работу. Но для кого-то – это понятное желание не терять множество времени на учебу в университете. Что бы ни толкнуло вас на это решение, наша фирма готова помочь. Оперативно, профессионально и недорого сделаем диплом любого ВУЗа и любого года выпуска на подлинных бланках с реальными подписями и печатями.
Основная причина, почему люди покупают документ, – желание занять хорошую работу. Предположим, знания позволяют человеку устроиться на работу, однако документального подтверждения квалификации нет. В том случае если работодателю важно наличие “корочки”, риск потерять место работы достаточно высокий.
Приобрести документ университета можно в нашей компании в столице. Мы оказываем услуги по производству и продаже документов об окончании любых университетов России. Вы получите необходимый диплом по любой специальности, любого года выпуска, включая документы образца СССР. Гарантируем, что в случае проверки документа работодателями, никаких подозрений не возникнет.
Ситуаций, которые вынуждают приобрести диплом очень много. Кому-то срочно нужна работа, а значит, нужно произвести хорошее впечатление на руководителя при собеседовании. Некоторые планируют попасть в престижную компанию, чтобы повысить собственный статус и в будущем начать свое дело. Чтобы не терять время, а сразу начать удачную карьеру, применяя врожденные таланты и приобретенные навыки, можно приобрести диплом прямо в онлайне. Вы сможете быть полезным в социуме, обретете денежную стабильность в кратчайший срок- купить аттестат
Kxyufaita
| #
darkmarket url https://cyberdarkmarkets.com/ – dark web market urls
Sazrswd
| #
Купить документ ВУЗа можно в нашей компании. Мы оказываем услуги по продаже документов об окончании любых университетов России. Вы получите необходимый диплом по любым специальностям, любого года выпуска, в том числе документы образца СССР. Гарантируем, что при проверке документов работодателями, никаких подозрений не возникнет. click-boutique.ru/forum/?PAGE_NAME=profile_view&UID=48595
LightMaster
| #
Простой https://ufalex.ru язык о финансах.
TimothyTop
| #
Казино 7k — это современная платформа для азартных развлечений с разнообразным выбором игр. Здесь можно найти популяризированные видеослоты, настольные игры и игры в реальном времени с мастерскими дилерами. Интерфейс интуитивно понятный и легкий в использовании, что делает игру удобной и неповторимой. На 7k740.casino предусмотрены классные бонусы для гемблеров. В целом, казино предоставляет отличный опыт для каждого игрока.
Striker7398
| #
Новые https://bisstrahovanie.ru подходы в финансах.
Matthewciz
| #
Онлайн-Казино Вулкан Платинум — это новейшая платформа для онлайн-гемблинга с большим выбором игр. Здесь можно найти лучшие игровые автоматы, настольные игры и игры с живыми дилерами с квалифицированными дилерами. Дизайн пространный и простой в навигации, что делает игру комфортной и неповторимой. На shint4.ru предусмотрены бонусные бонусы для гемблеров. В целом, казино предоставляет неповторимый опыт для каждого игрока.
Jerrymop
| #
cocaine esports gay
TimothyCob
| #
cocaine esports heroin
PhantomFang
| #
Малоизвестные фишки https://flagman-kpk.ru финасов.
Sazrsxi
| #
купить диплом электрогазосварщика
FNDaviditabe
| #
darkmarket https://onion-dark-market.shop/ – bitcoin dark web
OmegaV4
| #
Заинтересовался финансами. Решение здесь: https://credit-pushkino.ru
mostbet_edOr
| #
mostber https://www.agility.forum24.ru/?1-0-0-00000756-000-0-0-1742360323 .
1win_fyPt
| #
официальный сайт 1win https://agility.forum24.ru/?1-0-0-00000755-000-0-0-1742359870/ .
WilliamIRrutty
| #
darknet websites https://asap-market-onion.com/ – darknet site
SilverOverlord
| #
Где смотреть актуальные данные о финансах? https://uksojuz.ru
1win_pbEn
| #
descărca 1win https://www.1win5002.ru .
Ralphtriny
| #
Odwiedzilem strone ??? liczac na, ze przetestuje dobra strone, ale to po prostu porazka! Interfejs starodawny, menu niewygodne, a rozgrywka ciagle sie zawieszaja. Zbyt duzo banerow, czas ladowania jest zbyt dlugi, a pomoc nie reaguje na prosby. Informacje na stronie sa chaotyczne i bezuzyteczne. Wydaje sie, ze strona zostala porzucona. Polecam przeznaczyc czas na rzeczywiscie wiarygodne strony!
Kxyufaita
| #
darkmarkets https://cyberdarkmarkets.com/ – darknet drug links
LightCode
| #
https://lombard-garant-ug.ru Чек-лист для работы с финасами.
Byte5909
| #
Давайте обсудим о финансах: https://car-leazing.ru
JefferyEvilm
| #
rolex for women
https://systemcheck-wiki.de/index.php?title=Benutzer:MonteCanales07
JeffreyVet
| #
Bukmacher oraz kasyno internetowe Mostbet to jeden z najdłużej działających serwisów hazardowych w sieci z ofertą dla Polaków, gdyż został założony jeszcze w 2009 roku. Platforma może pochwalić się posiadaniem aktywnej licencji od Curacao, która gwarantuje bezpieczeństwo. Gracze będą mogli spotkać tutaj ponad 3000 zróżnicowanych gier hazardowych, które zostały podzielone na automaty, gry stołowe oraz gry na żywo. Nie zabraknie też interesującego dodatku w formie różnorodnych gier natychmiastowych, z czego najbardziej wyróżniają się gry natychmiastowe. Platforma Mostbet Casino udostępnia jednocześnie wiele zróżnicowanych tytułów od najbardziej popularnych dostawców oprogramowania, wśród których znajdują się takie uznane marki jak na przykład Play’n GO, Pragmatic Play, Evolution, Microgaming czy NetEnt. Do tego dochodzą różnorodne bonusy, promocje czy turnieje.
Bryanonefs
| #
They consistently exceed global healthcare expectations.
cytotec over the
A true champion for patients around the world.
Dragon_Master
| #
Нужны https://avtozalogkazan.ru реальные кейсы по финансам.
FNDaviditabe
| #
darknet markets onion https://firstdarkmarket.com/ – darkmarket
Light_Overlord
| #
Что нового в финансах? https://rieltorovich.ru.
WilliamIRrutty
| #
dark web drug marketplace https://darknetmarketsonion.shop/ – darknet drugs
Kxyufaita
| #
darknet drugs https://cannahomemarket.link/ – darkmarket
WillieViemy
| #
https://xnudes.app/
Phantom593
| #
https://dh068.ru Как сэкономить на финансах.
Red142
| #
Коллеги, поделитесь опытом по финансам! https://dengicapital.ru
1win_eaPt
| #
1вин вход с компьютера http://www.agility.forum24.ru/?1-0-0-00000755-000-0-0-1742359870 .
mostbet_otOr
| #
mostbet kg mostbet kg .
Marlinzef
| #
whores esports whores
ThomasFek
| #
heroin esports porn
Jamesjenda
| #
юрист по уголовным делам
1win_jfEn
| #
1win молдова 1win молдова .
FNDaviditabe
| #
darknet links https://mydarkmarketlink.com/ – best darknet markets
WolfV7
| #
Рекомендую проверенный ресурс по финансам: https://antikvar63.ru
WilliamIRrutty
| #
dark market 2025 https://kingdomdarkmarketonline.com/ – darkmarket 2025
StephenEpige
| #
перейдите на этот сайт
оборудование для переработки пластика
Silver647
| #
Как сэкономить на финансах: https://stolichniy-coop.ru
Kxyufaita
| #
dark web market links https://kingdomdarknetdrugstore.com/ – darknet market links
BarryNip
| #
Миру могут угрожать вспышки неизвестных ранее болезней https://x.com/Fariz418740/status/1902696858473910276
SilverX9
| #
Подробный разбор финасов: https://dohodpk.ru
JefferyEvilm
| #
duplicate rolex
https://telegra.ph/Rolex-herrenuhr-gold-03-20
RickyNax
| #
купить права категории б
FNDaviditabe
| #
dark markets https://kingdom-marketplace.com/ – darkmarket url
LeroyRaign
| #
https://midler.ru/portfolio/
Blue_Hacker
| #
Оптимизация процессов через финансы: https://rbgarant.ru.
Miguelbok
| #
pin up
WilliamIRrutty
| #
darknet drug store https://idarknetmarket.com/ – dark web market urls
CoreyLal
| #
https://t.me/top_mobile_casino_2025/3 Остановитесь на нашем рейтинге мобильных казино онлайн — здесь только проверенные и надежные онлайн-платформы, которые уже завоевали доверие тысяч пользователей. Мы отобрали для вас ТОП-6 казино, предлагающих оптимальные условия для игры, щедрые бонусные программы и моментальные выплаты. Каждая платформа дарит бесплатные вращения за регистрацию, чтобы вы могли начать играть без риска и лишних вложений!
Thunder86
| #
Где смотреть https://saint-expert.ru статистику о финасах?
JefferyEvilm
| #
replicas rolex
https://telegra.ph/Rolex-sea-dweller-03-20
Richardaluby
| #
gay esports terrorist attack
CesarDam
| #
Free steam accounts http://t.me/s/freesteamaccountc
DriverBaile
| #
Сотрудник коммунальной службы подсказал медицинскую информацию. Организация похорон прошла без проблем. Спасибо за профессионализм.
AlphaX7
| #
Оптимизация процессов через финансы: https://vf-partner.ru
Reaper6618
| #
Исследование https://financgarant.ru финасов.
Bryanonefs
| #
Their private consultation rooms are a great addition.
how long do gabapentin take to work
A true asset to our neighborhood.
JefferyEvilm
| #
rolex kopie
https://e-webwiki.co.uk/index.php/Rolex_Yacht_Master_40_Rose_Gold
Dragon833
| #
Как https://quickzaim24.ru преуспеть в финансах?
NightZ5
| #
Преодоление трудностей https://finmoli.ru при изучении финасов.
RobertFal
| #
rolex oyster perpetual date blue
https://cannabis-cultivation.wiki/index.php?title=Rolex_69I
RobertFal
| #
rolex two tone women’s watch
https://orphelins-apocalypse.synology.me/mediawiki/index.php?title=Rolex_9X
BlueV1
| #
Финасы для компаний: https://aviscar.ru.
Steel_Nomad
| #
Где смотреть актуальные данные о финансах? https://skpknarod.ru
NewRetroCasino_ppel
| #
Зеркало Ретро Казино http://www.newretromirror.ru .
Voyager9386
| #
Мифы и правда о финасах: https://magazin583.ru
Fang6234
| #
https://primfermer25.ru Какие платформы использовать для финансов?
Robbiepew
| #
Easy piano sheet music free piano
RobertFal
| #
rolex tiffany blue 36mm price
https://telegra.ph/Datejust-white-03-20
DennisZen
| #
Klavier lernen noten kostenlos noten fur klavier zum ausdrucken
JefferyEvilm
| #
rolex daytona ice blue
https://morphomics.science/wiki/Rolex_Submariner_Date_126610lv
RobertFal
| #
how.much are rolex watches
https://championsleage.review/wiki/Rolex_66E
ThomasSlupt
| #
Noten von klavier noten vom klavier
nvblfurn
| #
https://rossahar.ru/rynok/pgs/igru_na_dvoih__ogon_i_voda.html играть в карты косынка c компьютером бесплатно и без регистрации
RobertFal
| #
rolex watch silver and blue
https://openbouffalo.org/index.php/User:Aretha53T2017
WolfVoyager
| #
https://cnbd-kredit-pod-zalog.ru Сравнительный анализ финасов.
RobertFal
| #
replica rolex watches for sale
https://reparatur.it/index.php?title=Benutzer:Kellye8981
Fang4987
| #
https://kpkd.ru Интерактивный курс по финансам.
maslyanie transformatori kypit_svKt
| #
масляные трансформаторы купить масляные трансформаторы купить .
VortexX1
| #
Конкретные шаги для работы с финасами: https://avtolombard02.ru
RobertFal
| #
https://indigenouspedia.com/index.php?title=Rolex_19Z
soglasovanie pereplanirovki kvartiri_xdMn
| #
перепланировка комнаты http://www.soglasovanie-pereplanirovki-kvartiry14.ru .
programmi 1s _gzKi
| #
программы 1с программы 1с .
Brandonsof
| #
накрутка лайков в Тик Ток бесплатно онлайн
Herbertdow
| #
find Kreativstorm
Harolddax
| #
Learn More Here
Kreativstorm Reviews
StormSpark
| #
Новые подходы в финансах: https://ums-rostov.ru
private jet charter flights
| #
Kudos, I value it!
https://niadd.com/article/1280000.html
1win_iyKi
| #
1вин бет официальный сайт 1вин бет официальный сайт .
Herbertdow
| #
here
Kreativstorm Training Program
1win_pdMi
| #
1 вин 1 вин .
Kxyufaita
| #
darkmarket url https://cannahomemarket.link/ – darknet site
RobertFal
| #
womens rolex sizes
https://www.ventura.wiki/index.php/Rolex_38H
Sazrrsz
| #
Приобретение подходящего диплома через надежную фирму дарит массу достоинств для покупателя. Данное решение дает возможность сберечь как продолжительное время, так и серьезные финансовые средства. Впрочем, только на этом выгоды не ограничиваются, преимуществ значительно больше.Мы можем предложить дипломы любой профессии. Дипломы производят на оригинальных бланках государственного образца. Доступная стоимость в сравнении с серьезными тратами на обучение и проживание. Заказ диплома об образовании из российского института станет выгодным шагом.
Быстро заказать диплом о высшем образовании: diploml-174.ru/diplom-med-kolledzha-kupit-2/
NightMaster
| #
С чего начать финасов? https://kpk-farvater.ru
mostbet_omei
| #
скачать mostbet http://www.ongame.forum24.ru/?1-18-0-00001219-000-0-0-1742360461 .
Robbiepew
| #
Piano sheet music free piano
MarkNOlaf
| #
onion dark website https://dark-web-versus.com – darknet site
Edwardwed
| #
Хотите списать долги? центр банкротство в казани законное освобождение от кредитных обязательств. Работаем с физлицами и бизнесом. Бесплатная консультация!
Thunder_Code
| #
https://antikvariatekb.ru Реальные примеры применения финансов.
StephenPhema
| #
Site-ul https://betsysrealfood.com este o resursa cu diverse articole in limba romana, acoperind subiecte precum sanatatea, nutri?ia, moda ?i stilul de via?a. Titlurile articolelor includ teme precum „?tiri de sanatate”, „?tiri pentru bebelu?i”, „?tiri de Medicina Alternativa” ?i „?tiri de via?a”. Site-ul este destinat publicului vorbitor de romana, interesat de un stil de via?a sanatos ?i re?ete de casa.
PhilipDycle
| #
https://football-today.uk/
NightX2
| #
Как сэкономить на финасах: https://lombardkazna.ru
FNDaviditabe
| #
darknet market list https://firstdarkmarket.com/ – darknet marketplace
RobertFal
| #
rolex for sale near me
https://telegra.ph/Womens-green-rolex-03-20
WilliamIRrutty
| #
darknet markets 2025 https://darknetmarketsonion.shop/ – dark web market links
Spark3723
| #
Креативное решение к финансам: https://urfirma-biko.ru
Kxyufaita
| #
dark web markets https://cannahomemarket.link/ – darknet markets 2025
profis
| #
Быстрая продажа и покупка https://profis.com.ua/ размещайте объявления о продаже товаров, поиске услуг, аренде недвижимости и работе. Простой интерфейс, удобный поиск, бесплатные публикации!
Night_Warden
| #
https://matkap35.ru Свежие тренды в финасах.
eurshallsautobodyensub
| #
Transform your car into a spectacular creation with Eurshall’s Auto Body’s outstanding tuning programs. Our experienced team excels at upgrading each vehicle to fulfill its full potential . Whether you’re aiming for agility or chic, our customized solutions ensure holistic results. Visit https://eurshallsautobody.com/ today to witness the enhancement and drive a vehicle that truly embodies your character .
RobertFal
| #
cheapest womens rolex
https://wiki.lanvollon.info/index.php/Rolex_78u
MarkNOlaf
| #
dark web market urls https://cannahomedarknetdrugstore.com/ – darkmarkets
1win_wrKi
| #
1win букмекер http://belbeer.borda.ru/?1-6-0-00001555-000-0-0-1742473542/ .
RedX2
| #
Давайте обсудим о финансах: https://sport-pay.ru
1win_yjMi
| #
1win com https://taksafonchik.borda.ru/?1-14-0-00002041-000-0-0/ .
nedodjorse
| #
Koch Market гарантирует индивидуальный подход к каждому клиенту. Специализируемся на услугах дооснащение, полировка, тюнинг, детейлинг, оклейка. Обеспечиваем высокое качество работы. Ответим на вопросы и расскажем детальнее о действующих акциях. Ищете установка задней камеры? Koch-market.ru – здесь публикуем полезные статьи для автолюбителей. Цель Koch Market – увеличить доходность и обороты вашего бизнеса, а также сократить издержки. Оставьте на портале заявку, и мы в ближайшее время вам перезвоним. Задачи обсудим, запланируем работы и найдем лучшее решение.
mostbet_flei
| #
мостбет войти http://www.ongame.forum24.ru/?1-18-0-00001219-000-0-0-1742360461 .
FNDaviditabe
| #
darknet market https://mydarkmarketlink.com/ – darknet drug market
Sentinel7135
| #
Будущее финасов: https://premium196.ru
RobertFal
| #
rolex clone watches for men
https://flynonrev.com/airlines/index.php/Rolex_25N
WilliamIRrutty
| #
darknet site https://kingdommarketdarknet.com/ – bitcoin dark web
Night_Spark
| #
Какие изменения в финансах? https://mai-vc.ru.
Kxyufaita
| #
best darknet markets https://cannahome-darkmarket-online.com/ – dark web drug marketplace
RobertFal
| #
https://chrisophia.wiki/index.php/Rolex_77g
huxojeMaype
| #
Мечтаете встретить свою любовь? Тогда вам непременно стоит посетить Лилибум! Сайт предлагает простую регистрацию, открывает двери к новым знакомствам и дружбе. Стараемся безопасность вам и анонимность обеспечить. Вас ждет доброжелательная атмосфера. Ищете замужние желают познакомиться? Lilibum.ru – сайт, где можете прекрасно провести время. Порадует красивый и удобный интерфейс. Модераторы профили пользователей и фотографии внимательно проверяют. С Лилибум вы обязательно найдете свою половинку. Знакомьтесь, общайтесь и создавайте новые отношения!
PhilipDycle
| #
football
RobertFal
| #
rolex super clones for sale
https://semantische-richtlijnen.wiki/wiki/Rolex_62w
Steel167
| #
Как монетизировать https://kpk-proekt.ru финасов в бизнесе?
MarkNOlaf
| #
dark web market links https://kingdom-darkmarketplace.com – dark web marketplaces
DarkZ4
| #
Подкаст о финансах: https://atmosfera-fin.ru
FNDaviditabe
| #
dark market list https://mydarkmarketlink.com/ – darknet markets
WilliamIRrutty
| #
dark market onion https://asap-market-onion.com/ – darknet drugs
RobertFal
| #
https://telegra.ph/Gifts-for-roller-coaster-enthusiasts-03-20
Blue532
| #
Изучите https://roks22alt.ru этот источник о финасах.
AlphaWalker
| #
Основы финансов для новичков: https://msk-dt.ru
Kxyufaita
| #
dark web marketplaces https://kingdomdarknetdrugstore.com/ – darknet markets links
RobertFal
| #
rolex day date 41mm price
https://msgwiki.msgeducation.com/index.php/Rolex_92D
MarkNOlaf
| #
darkmarket link https://dark-web-versus.com – darkmarket url
Protocol7822
| #
Экспертный уровень в https://almaz-yar.ru финасах.
yinemgut
| #
Stakecasino.tech предоставляет полезную информацию. Stake Casino вас честными условиями и большим разнообразием азартных игр порадует. Сайт в применении довольно прост. Регистрация меньше минуты занимает. Проблем с выводом средств нет. Ищете stake casino промо? Stakecasino.tech – тут вы узнаете, как в Стейк казино начать играть. Платформа интуитивно понятный интерфейс предлагает, это делает для опытных игроков и новичков ее доступной. Стейк предоставляет специальные бонусы и акции. Консультанты службы поддержки доброжелательные и оперативно отвечают.
RobertFal
| #
rolex fake
https://wikistars5.com/index.php?title=Rolex_26D
Omega_Sentinel
| #
Где тренироваться финансам? https://furgongrad.ru
FNDaviditabe
| #
dark market list https://darkmarketdarkfox.com/ – dark market onion
RobertFal
| #
2023 prestige mega box
https://mediawiki.uedemersv.de/index.php?title=Benutzer:GloriaPeeples
WilliamIRrutty
| #
dark web market https://darknetmarketsonion.shop/ – darknet websites
DarkZ4
| #
Давайте обсудим о финасах: https://avtozaim1.ru
Sazrgpb
| #
Задумался а действительно можно купить диплом государственного образца в Москве, и был удивлен, все реально и главное официально!
Сначала серфил в сети и искал такие темы как: купить диплом мгюа, купить диплом о среднем образовании в абакане, купить диплом парикмахера цена, купить диплом перманентный макияж, купить диплом поступить в вуз, получил базовую информацию.
Остановился в итоге на материале proffdiplomik.com/sochi
Kxyufaita
| #
dark market list https://cannahome-darkmarket-online.com/ – dark web market
ShadowV3
| #
Подробный разбор финансов: https://buhgalter-vladikavkaz.ru
RobertFal
| #
datejust price
https://telegra.ph/Replica-vendors1-03-20
RobertFal
| #
https://disgaeawiki.info/index.php/User:Tangela0373
RobertFal
| #
aaa rolex
http://projectingpower.org:80/w/index.php/Rolex_78k
MarkNOlaf
| #
darknet market lists https://drdarkfoxmarket.com – dark web link
Blue771
| #
Что https://lombard-masterok.ru нового в финасах?
RedSlayer
| #
Вебинар о финансах: https://biznes-plani.ru.
FNDaviditabe
| #
darknet market list https://darkmarketdarkfox.com/ – dark market
WilliamIRrutty
| #
darkmarket link https://idarknetmarket.com/ – darkmarket url
RobertFal
| #
rolex datejust green dial price
http://wiki.wild-sau.com/index.php?title=Rolex_69e
Kxyufaita
| #
best darknet markets https://kingdomdarknetdrugstore.com/ – darknet markets onion
Byte6317
| #
Столкнулся с проблемой финасами. https://senexa.ru Решение здесь.
IronV2
| #
Мемы про финансы: https://autostrh.ru
RobertFal
| #
ss clone for sale
https://flynonrev.com/airlines/index.php/User:LavondaGilbreath
RobertFal
| #
nice rolex
https://www.openlongevityproject.org/index.php?title=User:UtaStapley9690
MarkNOlaf
| #
darknet market list https://drdarkfoxmarket.com – dark market
Jefferysmeap
| #
В Красной Поляне с радостью представляем вам сайт для знакомств с девушками 18+. Здесь вы сможете найти множество интересных предложений для общения и веселья, открывая для себя мир новых знакомств и ярких эмоций вместе с привлекательными дамами https://night24-krasnaya-polyana.com/
Iron_Hunter
| #
Читал прогнозы, что Финансы будет главным трендом. Соглашусь? https://finanexpert.ru
CyberWarden
| #
Нестандартный подход https://domoteka-omsk.ru к финасам.
FNDaviditabe
| #
darkmarket 2025 https://onion-dark-market.shop/ – dark web market links
RobertFal
| #
why is rolex watches so expensive
https://telegra.ph/Cheapest-place-to-buy-rolex-watches-03-20
RobertFal
| #
datejust rolex ebay
https://menuaipedia.menuai.id/index.php/Rolex_62z
fajoridox
| #
Copy General предоставляет широкий спектр качественных печатных услуг. Используем современное оборудование и технологии. Готовы ваши требования обсудить. К нам проявляют доверие множество частных, а также корпоративных клиентов по всему миру. Свяжитесь с нами прямо сегодня и убедитесь в качестве нашей работы. Ищете фотопечать? Copygeneral.ru – здесь представлены отзывы заказчиков, ознакомьтесь с ними. Типография работает без выходных и дает гарантию на отменное качество печати. Оплату можно произвести удобным способом. Стараемся ваши ожидания превзойти.
WilliamIRrutty
| #
dark market https://idarknetmarket.com/ – darkmarket list
RobertFal
| #
moissanite dealer near me
https://waselplatform.org/blog/index.php?entryid=208
nvblfurn
| #
https://veimmuseum.ru/news/pgs/ogon_i_voda_na_dvoih_onlayn_besplatno__uvlekatelnaya_igra_dlya_vseh.html какие игры можно поиграть дома на четверых
Kxyufaita
| #
onion dark website https://cannahomemarket.link/ – dark web market links
RobertFal
| #
1 1 watches
https://wiki.excito.org/index.php?title=User:AlfieRoldan56
ShadowZ7
| #
Коллеги, поделитесь опытом по финансам! https://abfns.ru
Light106
| #
https://finkorp.ru Коллеги, поделитесь опытом по финасам!
RobertFal
| #
milgauss for sale
https://telegra.ph/41mm-oyster-perpetual-03-20
Jefferysmeap
| #
Друзья, у нас для вас отличная новость! В Красной Поляне открылся сайт для досуга с девушками 18+. Теперь у вас есть возможность пообщаться с ними и провести время так, как вы мечтали. Откройте новый мир и наслаждайтесь уникальными моментами встреч https://night24-krasnaya-polyana.com/
RobertFal
| #
rolex deep sea-dweller 44mm
https://telegra.ph/Look-a-like-rolex1-03-20
MarkNOlaf
| #
darkmarket link https://cannahomedarknetdrugstore.com/ – darknet drug links
RobertFal
| #
superclone rolex
https://cannapedia.icu/index.php/Usuario:BryantFollansbee
RobertFal
| #
datejust 36mm vs 41mm
https://reparatur.it/index.php?title=Rolex_10S
Eagle_Sentinel
| #
Продвинутые техники финансов: https://mrparks.ru
RobertFal
| #
oyster pearl worth
https://telegra.ph/Ebay-oyster-perpetual-rolex-03-20
FNDaviditabe
| #
dark web market links https://firstdarkmarket.com/ – darknet drugs
GhostV6
| #
Оптимизация процессов через финасов: https://ffr24.ru.
RobertFal
| #
rolex submariner white face
https://mediawiki.novastega.me/index.php/Rolex_16b
WilliamIRrutty
| #
darknet market links https://darknetmarketsonion.shop/ – dark web sites
RobertFal
| #
best place to buy rolex in miami
https://shikhadabas.com/2025/03/18/rolex-77z/
RobertFal
| #
rolex blue daytona
https://Ragnafrost.wiki/index.php/Rolex_16v
Kxyufaita
| #
bitcoin dark web https://cannahomemarket.link/ – onion dark website
Hunter5739
| #
Особенности реализации https://ctroidom74.ru финансов.
Cyber_Fang
| #
Лично мне помог этот ресурс по финасам: https://mts-rus.ru
MarkNOlaf
| #
darkmarket https://kingdomdarkmarketplace.com – dark web sites
Bobbyinhex
| #
размещение рекламных конструкций светящиеся буквы
RobertFal
| #
submariner rolex band
https://reparatur.it/index.php?title=Rolex_46c
DonaldPap
| #
оформление вывесок изготовление рекламных конструкций спб
FNDaviditabe
| #
darknet drugs https://mydarkmarketlink.com/ – darknet websites
Ghost_Slayer
| #
Что не https://prof25.ru пишут в учебниках с финансами.
Matthewvet
| #
медицинские справки санкт петербург https://kupit-spravku-spb.ru
WilliamIRrutty
| #
dark web market links https://asap-market-onion.com/ – dark market onion
CyberZ1
| #
Свежие тренды в финансах: https://crowdautoinvest.ru
RobertFal
| #
https://Ragnafrost.wiki/index.php/Utilizador:CorinneDespeissi
WolfV6
| #
Ошибки новичков в финасах: https://family-stav.ru
RobertFal
| #
best online rolex dealer
https://andyfreund.de/wiki/index.php?title=Rolex_49z
Cazrnku
| #
Привет!
Без присутствия диплома очень нелегко было продвинуться вверх по карьерной лестнице. В настоящее время документ не дает гарантий, что удастся получить привлекательную работу. Намного более важное значение имеют навыки и знания специалиста и его опыт. Именно из-за этого решение о заказе диплома можно считать мудрым и рациональным. Приобрести диплом о высшем образовании tricityfriends.com/read-blog/30370
Charlesviank
| #
https://promocode.game/cs2/csfail/
Dnrtfwj
| #
Где заказать диплом по нужной специальности?
Мы изготавливаем дипломы любых профессий по приятным тарифам. Мы готовы предложить документы техникумов, которые находятся на территории всей РФ. Можно приобрести диплом от любого заведения, за любой год, в том числе документы старого образца. Дипломы и аттестаты печатаются на “правильной” бумаге самого высокого качества. Это позволяет делать государственные дипломы, которые не отличить от оригинала. Документы будут заверены всеми обязательными печатями и штампами. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы документы были доступными для большого количества граждан. diplomdoc.ru/kupit-attestat-shkoli-7
RobertFal
| #
rolex 1992
https://telegra.ph/Gold-mens-rolex-datejust1-03-20
RobertFal
| #
superclone watch
https://telegra.ph/How-much-are-rolex-watches-worth-03-20
Kxyufaita
| #
darknet market list https://kingdomdarknetdrugstore.com/ – darkmarket 2025
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://17metrov.ru/
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://1domovoy.ru/
RobertFal
| #
https://haloleagues.com/rolex-38h/
1win_eeei
| #
wan win wan win .
MarkNOlaf
| #
dark market url https://cannahomedarknetdrugstore.com/ – darknet links
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://1eve1.ru/
Thunder_Nomad
| #
Что лучше выбрать в финасах? https://prostozaimi.ru
PhantomWarden
| #
Как https://alfaexpert32.ru сделать финансы?
Dark326
| #
Столкнулся с проблемой https://kredtaxi.ru финансами. Решение здесь.
finuvTus
| #
Сомово – компания, которая большой ассортимент мебельной продукции предоставляет. Мы рады общению с нашими дорогими клиентами. Стараемся вам исключительно лучшее дать. Одна из главных целей – формирование длительных отношений. Somovo-Ищете купить прихожие в Москве? mebel.ru/catalog/prikhozhii – здесь можно купить прихожие недорого с оперативной доставкой. При возникновении вопросов, позвоните нам по телефону, который на ресурсе указан. С радостью поможем с выбором и детали заказа подскажем. В интернет-магазине «Сомово» найдется товар, который необходим именно вам!
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://2052285.ru/
ForrestVag
| #
кракен маркет – кракен вход, kraken ссылка
Michaelmiz
| #
Автоматизация ПДС также включает поддержку реестра НПФ, что позволяет фондам оперативно обновлять данные об участниках и их накоплениях. Система веб-доступа к счетам НПФ обеспечивает участникам возможность отслеживать состояние своих счетов в режиме реального времени, что повышает доверие к фонду. Бэк офис НПФ
RobertFal
| #
blue rolex submariner
https://syria-wiki.org/index.php?title=Rolex_61S
elektrokarnizi dlya shtor_hxot
| #
электрокарнизы цена электрокарнизы цена .
hMustafaVep
| #
Заказала пионы подруге, она сказала, что это лучший подарок!
Пионы томск
FNDaviditabe
| #
best darknet markets https://firstdarkmarket.com/ – dark market 2025
RobertFal
| #
https://telegra.ph/Rolex-lady-datejust-on-wrist-03-20
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://2204000.ru/
WilliamIRrutty
| #
darknet websites https://asap-market-onion.com/ – darknet drug links
mostbet_vsmn
| #
служба поддержки мостбет номер телефона http://www.mostbet782.ru .
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://22058.ru/
nvblfurn
| #
https://suvredut.ru/img/pgs/igru_bez_interneta_besplatno__luchshie_variantu_dlya_vashego_ustroystva.html цветные линии шарики 98 играть онлайн
EagleX7
| #
Преимущества разных подходов к финасам: https://algcons.ru
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://2231109.ru/
RobertFal
| #
mens knock off watches
https://kreosite.com/index.php/User:MillardQ22
Kxyufaita
| #
darknet market list https://darkfoxmarketplace.com/ – bitcoin dark web
Overlord4586
| #
Что лучше выбрать в финансах? https://afr54-ipoteka.ru
Thunder595
| #
Нестандартный подход к финансам: https://myasnaya-zastava.ru
ThomasVep
| #
Букет подарил море эмоций, спасибо за красоту!
букет цветов томск
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://2k-metalwork.ru/
1win_gtei
| #
1вин вход 1вин вход .
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://337700.ru/
Sazrbzd
| #
Добрый день!
Приобрести диплом университета по выгодной цене можно, обращаясь к проверенной специализированной компании. Приобрести диплом о высшем образовании: diplom-top.ru/kupit-diplom-farmatsevta-10/
MarkNOlaf
| #
darknet sites https://kingdomdarkmarketplace.com – dark markets 2025
Trefjmg
| #
Приобрести документ о получении высшего образования вы имеете возможность в нашем сервисе. diplomd-magazinp.ru/kupit-diplom-moskva-4-2
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://33panorama.ru/
Pioneer4647
| #
Продвинутые техники финасов: https://troika-zaim.ru
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://3855154.ru/
RobertFal
| #
https://wiki.radiomurf.com/wiki/User:Arden15M27731851
GhostX7
| #
Конкретные шаги для работы с финансами: https://finconstrall.ru
FNDaviditabe
| #
darknet markets onion address https://onion-dark-market.shop/ – dark websites
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://3909102.ru/
EagleCode
| #
Экспертный уровень в финансах: https://kredit19.ru
WilliamIRrutty
| #
dark web sites https://idarknetmarket.com/ – darknet marketplace
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://417-017.ru/
mostbet_symn
| #
mostbet игры https://mostbet782.ru/ .
Kxyufaita
| #
darknet market list https://cannahome-darkmarket-online.com/ – dark websites
1win_kg_xhKn
| #
win 1 https://1win823.ru/ .
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные статьи и советы — https://4masterss.ru/
Spark3371
| #
Эксклюзивное интервью с экспертом https://arifmetika-mkk.ru по финасам.
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://565606.ru/
1win_nfei
| #
1wln http://1win822.ru/ .
Russellovank
| #
Casino Nomini http://nomini-casino.ru
RedV4
| #
Как войти https://fif124.ru в тему финансов?
1win_kg_ltKn
| #
1win.kg 1win.kg .
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://740-789.ru/
MarkNOlaf
| #
dark web market links https://dark-web-versus.com – dark market
Red480
| #
Как сэкономить на финансах: https://aksay-rich.ru
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://74evakuator.ru/
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://7832206.ru/
Blade2195
| #
Будущее финасов: https://el-1.ru
nvblfurn
| #
http://svidetel.su/jsibox/article/index.php?ogon_i_voda_onlayn___uvlekatelnoe_prikluchenie_dlya_vseh.html пасьянс гольф играть бесплатно и без регистрации
FNDaviditabe
| #
darknet markets onion https://darkmarketdarkfox.com/ – darknet markets url
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://7lestnic.ru/
WilliamIRrutty
| #
dark web sites https://darknetmarketsonion.shop/ – dark web drug marketplace
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://9002781.ru/
mostbet_ahmn
| #
мосбет https://www.mostbet782.ru .
1win_kg_jyKn
| #
1 цшт http://1win823.ru .
Blade5911
| #
Конкретные шаги для работы с финансами: https://carcash-vdk.ru
cevinuUnins
| #
МВПОЛ услуги по выполнению промышленных полов предоставляет. Наша команда состоит из грамотных сотрудников, которые применяют новейшее оборудование, а также инструменты. Все работы совершаются в сжатые сроки. Ищете полимерные полы цена м2? Mvpol.ru – тут есть возможность посмотреть условия сотрудничества и стоимость услуг. На портале наши объекты представлены. Мы тщательно контролируем то, чтобы любой наш проект был закончен в соответствии с высочайшими стандартами. Мы знаем, что надо заказчику. У вас останутся приятные впечатления от сотрудничества с компанией МВПОЛ.
Kxyufaita
| #
dark web market urls https://darkfoxmarketplace.com/ – dark web markets
Markcen
| #
Покупка квартиры без рисков? Полезные статьи и советы — https://999555.ru/
GhostBlade
| #
Мне самому помог этот ресурс по финансам: https://ifa-invest.ru
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://a1realtyspb.ru/
Blue_Overlord
| #
ТОП-10 ресурсов по финасам: https://pravoved59.ru.
MarkNOlaf
| #
dark web marketplaces https://cannahomedarknetdrugstore.com/ – darknet markets 2025
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://abmodul.ru/
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://abrikos-krsk.ru/
Blue314
| #
Всем привет! Может кто в https://pawntrade.ru курсе, где найти информацию о финансах?
Jariorpfx
| #
Где купить диплом специалиста?
Мы предлагаем выгодно и быстро приобрести диплом, который выполняется на бланке ГОЗНАКа и заверен мокрыми печатями, штампами, подписями официальных лиц. Данный документ способен пройти лубую проверку, даже с применением профессионального оборудования. Достигайте цели быстро с нашим сервисом.
Купить диплом о высшем образовании diplomt-v-chelyabinske.ru/kupit-diplom-motorista/
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://azovcity-kuban.ru/
FNDaviditabe
| #
onion dark website https://firstdarkmarket.com/ – darknet markets onion address
Iariorbvr
| #
Купить документ университета вы имеете возможность у нас в Москве. usawc.org/submit-mailbag-item-for-magazine
Lazrfgb
| #
Приобрести диплом об образовании!
Мы можем предложить дипломы любой профессии по приятным тарифам— diplom-kaluga.ru/kupit-diplom-v-novosibirske-20/
StormVoyager
| #
Видеоуроки по финасам: https://spkk-kazna.ru.
WilliamIRrutty
| #
darknet market https://asap-market-onion.com/ – darkmarkets
Night777
| #
Как применять финансы в бизнесе? https://kpkveles.ru
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://b-import.ru/
soyt moskva_xbPa
| #
провести соут москва провести соут москва .
soyt moskva_fpkn
| #
заказать соут в москве заказать соут в москве .
Michaelsat
| #
hop over to these guys
galaxyswapper
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://baget-mos.ru/
Kxyufaita
| #
dark market link https://cannahome-darkmarket-online.com/ – darknet markets onion
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://baltimkom.ru/
BarryNip
| #
Миру могут угрожать вспышки неизвестных ранее болезней https://x.com/Fariz418740/status/1902696858473910276
ShadowGuardian
| #
Интерактивный курс по финансам: https://b-act.ru
Alpha_Pioneer
| #
Технические нюансы финасов: https://lombard-altyn.ru
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://baltstirol39.ru/
MarkNOlaf
| #
dark web drug marketplace https://dark-web-versus.com – darknet markets
Jasonquinc
| #
Доставка в раннее время прошла без проблем.
букет невесты
lacohiflops
| #
СанОбработка – компания, которая для выполнения чистоты и безопасности вашего дома предоставляет комплексные решения. Гарантируем индивидуальный подход к каждому клиенту. С удовольствием вам поможем. Вы останетесь довольны приемлемыми ценами и качеством обслуживания. Ищете дезинфекция вентиляции Тюмень? Sanobrabotkarf.ru – здесь представлены отзывы наших клиентов. Персонал вежливый и компетентный. Специалисты детально объяснят все этапы работы и ответят на вопросы. Они используют сертифицированные препараты и современные технологии. Защитите собственное жилище уже сегодня!
Cazrsku
| #
Мы изготавливаем дипломы любых профессий по приятным тарифам. Купить диплом бурильщика — kyc-diplom.com/diplomy-po-professii/kupit-diplom-burilshhika.html
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://basban31.ru/
LightV5
| #
Вебинар о https://doveriecredit.ru финансах.
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://bastion-bezheck.ru/
FNDaviditabe
| #
darkmarkets https://darkmarketdarkfox.com/ – dark market url
WilliamIRrutty
| #
darknet drug market https://kingdomdarkmarketonline.com/ – darkmarket url
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://batstroimat24.ru/
DarkNomad
| #
https://berirubli.ru Мифы и правда о финасах.
Red955
| #
Чек-лист https://salon-novosel.ru для работы с финансами.
RobinIrono
| #
Хотите перекусить вкусно? купить Хлеб для сэндвичей питу, шаверму, донер, гирос и тортилью. Ароматная свежая выпечка, мясо на гриле и фирменные соусы. Отличный выбор для перекуса, обеда или вечеринки.
Kxyufaita
| #
dark market https://cannahome-darkmarket-online.com/ – dark web market links
Pioneer5691
| #
Что вы думаете https://oboi42.ru этот ресурс о финансах?
WolfOverlord
| #
Эволюция развития финасов: https://zaim-vkarmane.ru.
MarkNOlaf
| #
best darknet markets https://cannahomedarknetdrugstore.com/ – dark markets 2025
NightV2
| #
Шутки про финансы: https://sidimdoma35.ru
FNDaviditabe
| #
dark market 2025 https://darkmarketdarkfox.com/ – dark web market
WilliamIRrutty
| #
dark web market urls https://darknetmarketsonion.shop/ – dark web market list
Hunter2785
| #
Согласно исследованиям, лучший https://ciframix.ru источник о финасах.
SteelWarden
| #
Смешные случаи в финансах: https://investassistant.ru
nvblfurn
| #
http://tankfront.ru/axis/pages/?igru_bez_ustanovki_besplatno__naslazghdaytes_razvlecheniyami_onlayn.html занятие с детьми 5 6 лет
Kxyufaita
| #
darkmarket url https://kingdomdarknetdrugstore.com/ – dark market link
DragonNomad
| #
Новые https://lombard-club.ru подходы в финансах.
Fang5293
| #
5 главных ошибок при https://sakhtools.ru работе с финасами.
MarkNOlaf
| #
darknet site https://kingdomdarkmarketplace.com – dark market 2025
tipografiya
| #
заказать журналы в типографии заказать книгу в типографии
IronWalker
| #
Полезные сервисы https://mkk-pfk.ru для работы с финансами.
FNDaviditabe
| #
darknet links https://mydarkmarketlink.com/ – dark market
WilliamIRrutty
| #
dark market 2025 https://asap-market-onion.com/ – darknet markets
Shadow373
| #
Что не пишут в учебниках с финасами: https://partner31.ru
ShadowSpark
| #
Ответы на частые вопросы о финансах: https://prliz.ru
Wallacesoolo
| #
типография печать табличка пвх
Lonniezem
| #
Эта клиника предлагает комфортное и безопасное лечение зубов детям, подробности тут https://500px.com/p/tschelkunchik?view=photos
Kxyufaita
| #
dark market https://cannahomemarket.link/ – darknet drugs
Lazrlad
| #
Где заказать диплом специалиста?
Приобрести диплом университета по доступной стоимости вы сможете, обращаясь к проверенной специализированной компании.: peoplediplom.ru
AlphaZ4
| #
https://kpksssr.ru Список инструментов по финасам.
Eagle_Warden
| #
Успешные кейсы применения https://kpk-lider.ru финансов.
WilliamDix
| #
Мы изготавливаем дипломы любой профессии по доступным ценам. Дипломы изготавливаются на настоящих бланках Заказать диплом института diplomidlarf.ru
MarkNOlaf
| #
darknet markets links https://kingdomdarkmarketplace.com – dark markets 2025
1win_pwPa
| #
1win казино https://www.1win810.ru .
Sazrymn
| #
Заказать документ ВУЗа вы сможете в нашем сервисе. Мы оказываем услуги по продаже документов об окончании любых университетов России. Вы сможете получить диплом по любой специальности, любого года выпуска, в том числе документы Советского Союза. Даем гарантию, что при проверке документов работодателем, никаких подозрений не появится. bookkarocallgirls.copiny.com/question/details/id/1069080
mostbet_agpr
| #
служба поддержки мостбет номер телефона http://www.mostbet783.ru .
Jamesflums
| #
Розыгрыш сертификатов OZON и WB – участвуй бесплатно!
Хотите получить подарочный сертификат Ozon или подарочный сертификат Wildberries совершенно бесплатно? Тогда подписывайтесь на наш Telegram-канал и участвуйте в розыгрышах!
В нашем канале регулярно разыгрываются сертификаты Ozon и Wildberries номиналом от 500 до 5000 рублей. Получить их можно легко:
1.Подписывайтесь на канал
2.Участвуйте в розыгрышах, выбирая билеты
3. Ожидайте результаты – победители определяются случайным образом!
Подарочные карты Ozon и Wildberries – это удобный способ оплатить любые покупки на популярных маркетплейсах. Участвуйте в розыгрышах и выигрывайте!
Присоединяйтесь прямо сейчас: Розыгрыш сертификатов OZON WB
Ghost139
| #
Как сэкономить на финансах: https://carzaym24.ru
1win_edPa
| #
игра 1вин игра 1вин .
mostbet_irpr
| #
mostbet casino http://mostbet783.ru .
nvblfurn
| #
https://www.colonoscopy.ru/PHPMailer/?igroutka__besplatnue_onlayn_strelyalki_dlya_malchikov___zahvatuvaushie_igrovue_priklucheniya_zghdut_vas.html огонь и вода 4 в хрустальном храме
mostbet_amSt
| #
мосбет mostbet784.ru .
FNDaviditabe
| #
tor drug market https://onion-dark-market.shop/ – dark web drug marketplace
Code6243
| #
Профессионалы рекомендуют эти ресурсы по финасам: https://protos39.ru
WilliamIRrutty
| #
dark market url https://asap-market-onion.com/ – darkmarket list
mostbet_bfSt
| #
mostbet kg скачать https://mostbet784.ru .
Fang5453
| #
Мифы и правда о финансах: https://kristelin.ru
1win_hsPa
| #
1win kg 1win kg .
mostbet_jupr
| #
мос бет https://mostbet783.ru/ .
Kxyufaita
| #
darknet markets 2025 https://darkfoxmarketplace.com/ – tor drug market
Dianna
| #
I always spent mmy half an hour to read this
blog’s content everyday along with a mug of coffee. https://Distribuidorafp.com.co/bdmbet-casino-evaluations-engage-in-new-games-through-your-account-and-secure-up-to-hours-of-fun-terms-and-more/
Voyager4790
| #
Что вы думаете этот https://alpari-cabinet.ru ресурс о финансах?
DarkX4
| #
Как думаете, где https://finanspg.ru лучше всего понять финасов?
DavidViedy
| #
B-52-ואדים הנהן בשלווה, מסכים להצעה “לטפל” – וגם פולינקה-הוא קרץ לנסטה – אני ואדיה! – הוא הציג את עצמו, לחץ ידיים עם החבר ‘ ה תזיין את התוכנית כמובן, הנה חמישה כוכבים, אתה לא יכול להגיד כלום. ומה אנחנו עושים במצבים האלה? נכון-רווהם. מתלונן, מתחנן. – לא, read this article
mostbet_trSt
| #
поддержка мостбет https://www.mostbet784.ru .
MarkNOlaf
| #
darknet markets url https://kingdomdarkmarketplace.com – darknet markets 2025
Liwuhssoomo
| #
На сайте http://ammely.ru представлены интересные, увлекательные статьи, которые точно понравятся женской аудитории. Опубликован контент о моде, красоте, здоровом образе жизни. Также имеются и полезные рецепты, которые идеально подойдут на каждый день либо на праздник, если внезапно нагрянули гости. Есть увлекательные рекомендации на тему дома, дизайна. Вам будет интересно узнать и о звездах, о том, как лучше и веселей провести праздник.
LesterZog
| #
https://plusgestio.com/pag/1xbet_today_promo_code_bonus_up_to_130.html
Dragon_Overlord
| #
Как https://elecland.ru реализовать финансы?
Lonniezem
| #
Для бизнеса: ускоренное получение лицензии за 14 дней с полной гарантией, подробнее по ссылке http://prsync.com/ilya-filippov/
FNDaviditabe
| #
darknet market list https://firstdarkmarket.com/ – dark market 2025
Dark874
| #
Оптимизация процессов через финасов: https://max-lombard.ru
WilliamIRrutty
| #
dark web marketplaces https://asap-market-onion.com/ – darknet markets links
WanuxnelalI
| #
На сайте https://xn—-8sbkf3cqel4a6c.xn--p1ai/ рассчитайте стоимость такой услуги, как ДТФ-печать на сувенирной продукции, текстиле. Все изображения отличаются безупречным качеством и цветопередачей, высокой детализацией. При необходимости вы сможете получить профессиональную консультацию. Главное направление деятельности компании – это DTF-печать на повседневной, корпоративной одежде, включая худи, футболки, толстовки. Все заказы выполняются с применением инновационных материалов, качественного оборудования.
Byte1878
| #
Какие изменения в финансах? https://pic-capital.ru
wplay
| #
whoah this weblog is wonderful i really like studying your posts.
Keep up the great work! You already know, lots of persons are searching around for this information, you can aid them greatly.
Nord-West
| #
Сияние вашего авто начинается здесь! Nord-West детейлинг работает с автомобилями премиум-класса уже более 10 лет. Оклейка кузова, полировка и керамика высшего качества доступны в Великом Новгороде. Доверьте свой автомобиль профессионалам.
Kxyufaita
| #
darknet markets onion https://cannahomemarket.link/ – darknet websites
Silver992
| #
Практическое применение https://mihbat.ru финансов.
Protocol2961
| #
Мой опыт показывает, https://zolotaya-pora.ru что по финасам лучше всего читать здесь.
wplay
| #
I’d like to thank you for the efforts you have put in penning this site.
I am hoping to view the same high-grade blog posts
from you in the future as well. In fact, your creative writing
abilities has encouraged me to get my own blog now 😉
MarkNOlaf
| #
darknet markets url https://kingdomdarkmarketplace.com – darknet links
jewejDyeds
| #
Calypso – комфортный для аренды яхт сервис. Наш подход ориентирован на удовлетворение персональных потребностей каждого клиента. Мы готовы обеспечить высочайший уровень сервиса и безопасность. Ваше путешествие на яхте станет невероятным! Ищете аренда катера Сочи? Sochi.calypso.ooo – тут можете с условиями заказа ознакомиться. На сайте вы найдете самые выгодные предложения. Поможем вам яхту, выбрать, которая вашим пожеланиям и требованиям соответствует. Присоединяйтесь к Calypso. Позвольте нам ваши мечты о безупречном отдыхе в реальность превратить!
NightX1
| #
С чего начать финансов? https://sopranocapital.ru
FNDaviditabe
| #
darknet marketplace https://darkmarketdarkfox.com/ – onion dark website
Look At This
| #
Hello there! Do you use Twitter? I’d like to follow you if that would be ok.
I’m absolutely enjoying your blog and look forward to new posts.
OmegaSentinel
| #
5 главных https://dengi-ontime.ru ошибок при работе с финасами.
WilliamIRrutty
| #
onion dark website https://asap-market-onion.com/ – darkmarket link
Vortex158
| #
Как сделать финансы? https://zaymlombard-top.ru
Kxyufaita
| #
dark web markets https://darkfoxmarketplace.com/ – darknet marketplace
Thunder601
| #
ТОП-10 ресурсов по финансам: https://777uspeh.ru
Alpha681
| #
Обнаружил, что по финасам лучше всего смотреть здесь: https://lombard-chronograph.ru
امنیت سایت
| #
Howdy! I could have sworn I’ve been to this web site before
but after going through many of the articles
I realized it’s new to me. Nonetheless, I’m definitely
delighted I found it and I’ll be bookmarking it and checking back regularly!
CyberProtocol
| #
Видел прогнозы, что Финансы будет главным трендом. Возражения? https://reteilbank.ru
MarkNOlaf
| #
dark web link https://cannahomedarknetdrugstore.com/ – darknet markets url
FNDaviditabe
| #
dark markets https://onion-dark-market.shop/ – dark web market list
WilliamIRrutty
| #
darknet marketplace https://kingdommarketdarknet.com/ – darknet websites
DarkOverlord
| #
https://a-leksmebel.ru Что нового в финасах?
аркада официальный сайт
| #
Nice post. I learn something new and challenging on blogs I stumbleupon everyday.
It’s always useful to read articles from other authors and practice
something from their web sites.
Omega_Master
| #
Как не сдаться при изучении https://insidersgroup.ru финансов.
DragonV1
| #
Эксперты https://rtl56.ru рекомендуют эти ресурсы по финансам.
Kxyufaita
| #
darkmarket list https://cannahome-darkmarket-online.com/ – darknet markets onion
DukeselSlorb
| #
На сайте http://kinomanhdc.online представлены фильмы в огромном многообразии. Перед вами как популярное кино, которое понравится всем без исключения, так и ожидаемые новинки, которые точно никого не оставят равнодушным. Есть возможность подобрать решение по определенной тематике: про вампиров, космос, подростков, девушек, любовь и многое другое. Здесь вы найдете и сериалы, киноленты за прошедшие годы. На сайте также представлены и мультфильмы для детей разного возраста, семейного просмотра. Все это вы сможете смотреть прямо сейчас и в режиме реального времени.
BlueX7
| #
Где смотреть https://vap-invest.ru статистику о финасах?
MarkNOlaf
| #
darkmarket link https://kingdom-darkmarketplace.com – darknet sites
constructive-m.ru
| #
Jetton Casino – это ваш билет в мир настоящего азарта и захватывающих побед. В нашем казино представлены только лучшие игровые автоматы, покер, рулетка и многое другое. Регистрируйтесь и начинайте выигрывать уже сегодня.
https://constructive-m.ru/ – это не просто казино, а целая вселенная для любителей азартных игр. Получайте кэшбэк, участвуйте в акциях и выигрывайте эксклюзивные призы. Гарантируем безопасные транзакции, а также удобные способы пополнения счета и вывода выигрышей.
Огромный ассортимент развлечений для всех любителей азартных игр.
Ежедневные подарки, кэшбэк и выгодные предложения для новых и постоянных игроков.
Быстрое пополнение счета и мгновенные выплаты без скрытых комиссий.
Эксклюзивные турниры с высокими призовыми фондами и шанс сорвать джекпот.
Начните игру в Jetton Casino и сделайте первый шаг к крупным победам.
игры с высоким RTP
| #
Добро пожаловать в Водка Казино — место,
где азарт встречается с невероятными бонусами и возможностями для выигрыша.
В нашем казино каждый игрок
найдет большой выбор слотов, карточных игр и рулетки, независимо от того, начинающий вы или опытный игрок.
Здесь вас ждет не только удовольствие от игры, но и множество выгодных предложений.
Все игры в Водка Казино
имеют высокий RTP, что увеличивает шансы на успешную игру.
Для вас мы подготовили интерактивные слоты, а также классические настольные игры, которые делают вашу игру более увлекательной и прибыльной.
Почему не начать прямо сейчас?
Водка Казино предоставляет игрокам мгновенную регистрацию и удобные способы пополнения счета, чтобы вы могли сосредоточиться на главном
— выигрыше!
У нас всегда есть для вас интересные бонусы, которые позволят вам начать играть с дополнительным капиталом.
Присоединяйтесь к Водка Казино, чтобы наслаждаться азартными играми с лучшими шансами на победу!
Зарегистрироваться можно
всего за несколько секунд.
Наслаждайтесь щедрыми бонусами сразу после регистрации.
Регулярные турниры и акции для тех, кто хочет увеличить
свои шансы на выигрыш.
Поддержка 24/7 для решения любых вопросов.
Играйте в любимые игры в любое время и в любом месте.
Начните выигрывать прямо сейчас и испытайте
удачу! https://vodka-777-spinwin.autos/
Byte8092
| #
Как сэкономить на финансах: https://credit-help32.ru
Red717
| #
Друзья, поделитесь опытом по финансам! https://hankukwan.ru
deneme bonusu veren siteler
| #
Kudos. Awesome stuff!
FNDaviditabe
| #
dark web market list https://darkmarketdarkfox.com/ – darknet markets links
WilliamIRrutty
| #
darknet markets url https://kingdommarketdarknet.com/ – darknet market
Hunter4783
| #
Повышение эффективности через финасов: https://an-biznespartner.ru
Kxyufaita
| #
dark market list https://cannahome-darkmarket-online.com/ – darkmarket link
DragonX6
| #
https://pts812.ru Вебинар о финансах.
StormOverlord
| #
Столкнулся с https://feniks-invest.ru проблемой финасами. Решение здесь.
VortexZ6
| #
Нюансы работы с https://maxlombard.ru финансами.
LesterZog
| #
https://nytalkradio.net/pag/1xbet_new_registration_promo_code_4.html
MarkNOlaf
| #
darknet site https://kingdom-darkmarketplace.com – dark market link
Aaronfek
| #
Срочная установка пластиковых окон за 3 дня с момента заказа — оставить заявку на замер можно здесь https://gitlab.com/AlexStone725
nvblfurn
| #
https://mytaganrog.com/media/madzghong_igrat_besplatno__zahvatuvaushee_prikluchenie_v_mir_drevney_igru.html игра красный шар 4 часть 1
siracajslusa
| #
К-ЖБИ гарантирует высочайшее качество продукции и строгое соблюдение сроков. Завод обладает гибкими производственными мощностями для выполнения заказов по чертежам заказчика. С удовольствием на все вопросы ответим, позвоните нам по номеру телефона. Ищете лестничные марши и бетонные площадки серии л лм лз и лп? Gbisp.ru – тут есть возможность заявку оставить. Укажите в форме ваш e-mail, имя и контактный номер. Далее нажмите кнопку «Отправить». Выполняем быструю доставку продукции. Обеспечиваем новейший уровень облуживания любому заказчику. К взаимовыгодным партнерским отношениям стремимся!
Aaronfek
| #
Средства индивидуальной защиты для работы в лаборатории – узнайте больше информации https://blacksocially.com/Janescience
FNDaviditabe
| #
darknet drugs https://mydarkmarketlink.com/ – darknet markets onion
Ghost873
| #
Топовый https://vipautosale.ru ресурс по теме финасов.
GeorgeWak
| #
Названы доказательства пребывания на Земле более высоких цивилизаций https://x.com/Fariz418740/status/1903334968513745235
WilliamIRrutty
| #
dark web link https://darknetmarketsonion.shop/ – darknet market lists
Storm586
| #
Что нельзя делать с финансами? https://kitleasing.ru
NightVoyager
| #
Как преуспеть https://rosa-rai.ru в финансах?
Jamesflums
| #
Церковь Валенсии Церковь в Валенсии – это не просто место отправления религиозных обрядов, это духовный центр, объединяющий верующих разных конфессий. Среди многообразия храмов и общин особое место занимают протестантские церкви, предлагающие альтернативный взгляд на христианство.
Kxyufaita
| #
dark web markets https://cannahome-darkmarket-online.com/ – best darknet markets
AlphaZ3
| #
Подкаст о финасах: https://inberi.ru.
Aurora casino онлайн
| #
Aurora Casino приглашает вас в мир высококлассных азартных игр и ярких эмоций. Каждый найдет здесь что-то по душе — от классических слотов до уникальных игр с реальными крупье. Мы также предлагаем различные бонусы и акции, которые сделают каждый момент игры еще более захватывающим.
Что делает https://auroracasino.live/ лучшим выбором для игроков? В Aurora Casino ваша безопасность и защита личных данных — наша первоочередная задача. Мы гарантируем прозрачные условия игры и мгновенные выплаты, чтобы вы могли наслаждаться выигрышами без задержек.
Когда вам лучше начать играть в Aurora Casino? Не откладывайте! Присоединяйтесь к нам и начните выигрывать прямо сейчас. Вот что вас ждет:
Большие бонусы и постоянные акции.
Мы гарантируем вашу безопасность и спокойствие, играя на нашей платформе.
Новинки и эксклюзивные игры.
В Aurora Casino вы всегда найдете яркие эмоции и шансы на большие выигрыши.
Sazrvrx
| #
Мы изготавливаем дипломы любой профессии по разумным ценам. Цена зависит от определенной специальности, года получения и образовательного учреждения. Всегда стараемся поддерживать для покупателей адекватную политику тарифов. Важно, чтобы дипломы были доступными для большого количества наших граждан. купить диплом на фармацевта
StormZ8
| #
Тесты по финансам: https://sokol-dv.ru
PhantomReaper
| #
Новые подходы https://aliansauto29.ru в финасах.
SteelX4
| #
https://arealdecor.ru Будущее финансов.
FNDaviditabe
| #
dark websites https://onion-dark-market.shop/ – dark web marketplaces
WilliamIRrutty
| #
darknet markets links https://kingdommarketdarknet.com/ – darknet market list
Jamesflums
| #
путешествие В мире, где горизонты становятся ближе, а мечты о приключениях — осязаемыми, наше агентство открывает двери в мир безграничных возможностей. Мы – ваш надежный компас в океане туризма, предлагая не просто путешествия, а мастер-туры, сотканные из ваших желаний и нашей экспертизы. Солнечная Турция, роскошные ОАЭ, загадочный Египет – мы подберем идеальное направление, соответствующее вашему вкусу. Оформление виз, организация трансфера, бронирование отелей и авиабилетов – все это мы берем на себя, чтобы вы могли полностью расслабиться и наслаждаться каждым моментом. Мечтаете о морском круизе или отдыхе в живописной Черногории? Хотите исследовать бескрайние просторы России? Мы поможем воплотить в жизнь самые смелые планы. Наша цель – сделать ваше путешествие незабываемым, комфортным и безопасным. Доверьтесь профессионалам, и ваш отпуск станет настоящим шедевром!
Kxyufaita
| #
darknet markets links https://cyberdarkmarkets.com/ – darknet markets onion address
Diplomi_sfKt
| #
Купить диплом любого ВУЗа!
Мы готовы предложить документы институтов, расположенных в любом регионе России.
diplomg-kurerom.ru/gde-kupit-diplom-texnikuma-5
mostbet_poMi
| #
mostbet mostbet .
AlphaX6
| #
Повышение эффективности https://fkarussia.ru через финасов.
EugeneKex
| #
печать листовок буклетов буклет офсетная печать
Henryged
| #
печать 3д наклеек https://pechat-nakleek-spb.ru
mostbet_eyEt
| #
mostbet casino https://taksafonchik.borda.ru/?1-14-0-00002042-000-0-0-1742473173 .
Vortex754
| #
Как вам этот ресурс https://kr-ankb.ru о финансах?
DerekRah
| #
печать логотипа на сувенирной продукции сувениры с печатью на заказ
mostbet_lrMi
| #
mostbest mostbest .
1win_vqoi
| #
wan win fanfiction.borda.ru/?1-3-0-00000125-000-0-0-1742475251 .
MarkNOlaf
| #
onion dark website https://cannahomedarknetdrugstore.com/ – darknet drug store
Mazrcdc
| #
Мы предлагаем дипломы любой профессии по приятным ценам. Стараемся поддерживать для покупателей адекватную ценовую политику. Важно, чтобы документы были доступными для большого количества граждан.
Заказ документа, подтверждающего обучение в университете, – это грамотное решение. Купить диплом любого ВУЗа: diplomt-v-chelyabinske.ru/kuplyu-diplom-o-visshem-obrazovanii-8/
mostbet_hfEt
| #
поддержка мостбет http://taksafonchik.borda.ru/?1-14-0-00002042-000-0-0-1742473173 .
ThunderWarden
| #
Профессионалы рекомендуют эти ресурсы по финансам: https://ivctt.ru
1win_ykoi
| #
1win.pro http://www.fanfiction.borda.ru/?1-3-0-00000125-000-0-0-1742475251 .
Cazraar
| #
Купить диплом о высшем образовании!
Мы можем предложить документы университетов, которые расположены на территории всей РФ. Дипломы и аттестаты выпускаются на бумаге самого высокого качества: opengroup.net/include/pgs/kupit_diplom_o_srednem_obrazovanii_29.html
DarkSlayer
| #
Изучите этот источник о финасах: https://kpkdelo.ru
wawembib
| #
Карамельная Фабрика мармелад, леденцы на палочке и другое предлагает. Каждый день наши карамелье шедевры создают. Купить вкусные и красивые сладости можно в нашем интернет-магазине. При возникновении вопросов, обращайтесь к нам, мы с удовольствием на них ответим. Ищете мастер класс по изготовлению карамели? Karamelfabrika.ru – здесь узнаете, как мы делаем карамель. Не останавливаемся на достигнутом и постоянно расширяем ассортимент. Проводим интересные мастер-классы, которые для детей станут источником радости и дадут возможность взрослым в детство ненадолго вернуться. Создаем для вас карамельное настроение!
mostbet_oiMi
| #
mostbet kg отзывы http://www.eisberg.forum24.ru/?1-0-0-00000327-000-0-0-1742579529 .
FNDaviditabe
| #
darknet markets onion address https://kingdom-marketplace.com/ – dark market
WilliamIRrutty
| #
darknet drug market https://kingdomdarkmarketonline.com/ – darknet drugs
mostbet_fvEt
| #
скачать мостбет официальный сайт http://www.taksafonchik.borda.ru/?1-14-0-00002042-000-0-0-1742473173 .
1win_vuoi
| #
1вин официальный http://fanfiction.borda.ru/?1-3-0-00000125-000-0-0-1742475251 .
Vortex407
| #
Приветствую. Может кто в курсе, где посмотреть информацию о финансах? https://sakhprokat.ru
Mazrftw
| #
Где заказать диплом специалиста?
Полученный диплом с нужными печатями и подписями целиком и полностью отвечает требованиям и стандартам, никто не отличит его от оригинала. Не следует откладывать собственные мечты на потом, реализуйте их с нашей помощью – отправьте быструю заявку на диплом уже сегодня! Диплом о среднем специальном образовании – легко! diplomus-spb.ru/nadezhnij-sposob-kupit-gotovij-attestat-bistro/
LightV1
| #
Практическое применение финасов: https://kpksodrug.ru
Davidzic
| #
Заказать пиццу Жаждете сочного итальянского наслаждения, не покидая Кемерово? Добро пожаловать в мир аппетитной пиццы, где каждый кусочек – это маленький праздник. Наша пицца на тонком, хрустящем итальянском тесте – это симфония вкусов, созданная из свежайших ингредиентов. Живете в Теплом ключе? Мы доставим ваш заказ прямо к вашей двери, быстро и аккуратно. А для тех, кто предпочитает забрать заказ самостоятельно, предусмотрена удобная опция самовывоза. Но это еще не все! Наша пиццерия предлагает не только восхитительную пиццу, но и сытные осетинские пироги, приготовленные по традиционным рецептам. Это идеальный выбор для тех, кто хочет попробовать что-то новое и аутентичное. Ищете идеальное место для празднования дня рождения? Мы дарим подарки именинникам, делая ваш особенный день еще более радостным и запоминающимся. Закажите пиццу прямо сейчас на нашем сайте и насладитесь непревзойденным вкусом!
Kxyufaita
| #
dark web markets https://kingdomdarknetdrugstore.com/ – darknet site
Cazreyf
| #
Заказать диплом любого ВУЗа мы поможем. Купить аттестат в Барнауле – diplomybox.com/kupit-attestat-v-barnaule
Alpha964
| #
Сравнение лучших ресурсов по финансам: https://lomkorona.ru
Volodyanaf
| #
dark market https://darkmarketpremium.com/ – dark market
MarkNOlaf
| #
dark market list https://kingdomdarkmarketplace.com – dark websites
Storm_Pioneer
| #
Как избежать проблем с финасами? https://real-credit24.ru
CyberZ5
| #
Как монетизировать финансы в бизнесе? https://cppnvrsk.ru
уборка после ремонта
| #
Безупречная чистота и комфорт в Вашем Доме:
Экспертные Услуги Клининговой Компании в Санкт-Петербурге городе на Неве
Измучились от нескончаемых забот по
уборке? Позвольте нам попечься о
чистоте вашего родного очага или офиса!
Наша клининговая компания в Санкт-Петербурге предлагает комплексные услуги, которые удовлетворят самые высокие
требования к качеству и сервису.
Доступные цены и прозрачные условия;
Высокое качество обслуживания и педантичное деталям;
Персональный подход к каждому клиенту;
Точное соблюдение сроки выполнения работ.
Не ждите, пока грязь и беспорядок станут катастрофой.
Подарите нам шанс возродить вашему пространству чистоту и порядок!
Узнайте всё о наших услугах и оставьте заявку на сайте
: уборка после ремонта
Toliknaf
| #
dark web market urls https://github.com/abacuslink4jjku/abacuslink – dark web link
FNDaviditabe
| #
dark market onion https://mydarkmarketlink.com/ – dark websites
Vortex709
| #
Полезные сервисы для работы с https://caratlombard.ru финансами.
клининговые компании спб
| #
Безупречная чистота и комфорт в Вашем Доме: Экспертные Услуги Клининговой Компании в Санкт-Петербурге СПб
Измучились от нескончаемых забот по уборке? Дайте возможность нам позаботиться о чистоте вашего дома или офиса! Наша клининговая компания в Санкт-Петербурге предлагает широкий спектр услуги, которые придутся по вкусу самые взыскательные требования к качеству и сервису.
Доступные цены и прозрачные условия;
Высокое качество обслуживания и внимание к деталям;
Персональный подход к каждому клиенту;
Чёткие сроки выполнения работ.
Не ждите, пока грязь и беспорядок станут катастрофой. Дайте нам шанс вернуть вашему пространству чистоту и порядок! Узнайте всё о наших услугах и оставьте заявку на сайте : https://cadpower.iitcsolution.com/bbs/board.php?bo_table=free&wr_id=269341
WilliamIRrutty
| #
dark web link https://kingdommarketdarknet.com/ – darknet websites
Silver_Guardian
| #
Изучите https://time27.ru этот источник о финасах.
Rabyitabe
| #
darknet websites https://github.com/darkwebsitesyhshv/darkwebsites – bitcoin dark web
Kxyufaita
| #
dark web market urls https://cannahome-darkmarket-online.com/ – darknet websites
Jariordme
| #
Где заказать диплом специалиста?
Наша компания предлагает быстро и выгодно купить диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями. Данный документ пройдет лубую проверку, даже при помощи профессионального оборудования. Достигайте свои цели быстро и просто с нашими дипломами. Приобрести диплом о высшем образовании! cosmeticsworld.org/read-blog/18084_svidetelstvo-o-brake.html
Xazrndi
| #
Купить диплом ВУЗа!
Мы предлагаем дипломы психологов, юристов, экономистов и других профессий по доступным тарифам. Вы приобретаете диплом через надежную и проверенную временем компанию. : diplom-kaluga.ru/kupit-diplom-mbi-o-visshem-obrazovanii-na-blanke-goznak-3/
GregoryTah
| #
Приобретите качественные купели для бани, или спа-зоны в нашем магазине! Мы предлагаем широкий ассортимент: традиционные модели из лиственницы и кедра, современные чаши с гидромассажем, варианты для небольших помещений https://kupeli-ural.ru/
Vortex125
| #
Мне самому помог этот ресурс по финансам: https://lombardsterling.ru
DonDonfaita
| #
darknet market https://github.com/nexusdarkrtv1u/nexusdark – darknet site
Volodyanaf
| #
darknet sites https://darkode-market.com/ – dark markets
Master9502
| #
https://spkk-optimist.ru Как реализовали финасов в компании.
Pingrutty
| #
dark web link https://github.com/aresonioncq0a7/aresonion – dark web market links
Thunder_Spark
| #
Доступно объяснено о финансах: https://avtozaem-moskva.ru
MarkNOlaf
| #
darkmarket link https://cannahomedarknetdrugstore.com/ – darknet sites
Vortex_Byte
| #
Может кто знает ориентируется в финасах? https://sebito.ru Где искать?
Donaldlaf
| #
dark web market links https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet market
FNDaviditabe
| #
dark websites https://kingdom-marketplace.com/ – dark web link
WilliamIRrutty
| #
darkmarket link https://kingdommarketdarknet.com/ – darknet markets
nvblfurn
| #
https://ma.by/docs/pages/?igru_na_73.html мультики про трансформеров для мальчиков 5 лет
Cyber_Voyager
| #
Предлагаю подискутировать о финансах: https://doer-group.ru
هایک ویژن
| #
سلام مقاله خوبی بود خرید هایک ویژن https://sites.google.com/view/hikvisiontehran/
Toliknaf
| #
dark market list https://github.com/abacuslink4jjku/abacuslink – darkmarkets
ThunderV1
| #
Глубокий анализ финансов: https://centrzaymov19.ru
Lonniezem
| #
Эксклюзивные ювелирные изделия с зубами динозавров – подробная информация здесь https://www.anobii.com/en/011a8d0efaca3880ef/profile/activity
Kxyufaita
| #
darknet markets onion address https://kingdomdarknetdrugstore.com/ – best darknet markets
Shadow_Hacker
| #
Секретные методы финасов: https://nfinanse.ru
Mazrvtn
| #
Где купить диплом по нужной специальности?
Мы оказываем услуги по продаже документов об окончании любых университетов России. Документы производят на подлинных бланках. ctivefanconnect.com/read-blog/14517_oficialnyj-attestat.html
Volodyanaf
| #
darknet markets url https://darkode-market.com/ – darknet sites
Rabyitabe
| #
darknet market lists https://github.com/darkwebsitesyhshv/darkwebsites – dark web sites
Pingrutty
| #
darknet market https://github.com/aresonioncq0a7/aresonion – darknet websites
MarkNOlaf
| #
dark web market urls https://cannahomedarknetdrugstore.com/ – darknet markets onion
ShadowNomad
| #
Продвинутые техники финасов: https://lombardych27.ru
dovezabanny
| #
Служба Эвакуации 911 предлагает качественные услуги. Мы оперативно и безопасно любой транспорт перевозим. Владеем новейшим автопарком эвакуаторов. Можете не сомневаться в ответственном подходе к вашей проблеме решению. Ищете Эвакуатор Пенза? Penza-evakuator.ru – сайт, где вы узнаете, какие виды техники мы готовы эвакуировать. Можем предложить наши услуги дешевле, чем у конкурентов. Работаем 24/7 без выходных. Помогаем клиентам порой в самых непростых ситуациях. Доверьте службе спасения 911 вашего авто транспортировку.
DonDonfaita
| #
darknet markets links https://github.com/nexusdarkrtv1u/nexusdark – darknet sites
Shadow_Guardian
| #
Коллеги, поделитесь опытом по финансам! https://tevsretni.ru
Sazrnuq
| #
Привет!
Купить диплом института по выгодной цене вы сможете, обратившись к надежной специализированной фирме. Заказать диплом: good-diplom.ru/kupit-diplom-tula-3/
Lazrxab
| #
Приобрести диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по приятным ценам— diplom-ryssia.com/kupit-meditsinskij-diplom-bistro-i-bez-lishnix-problem/
Nomad4060
| #
Мемы про финансы: https://ah19.ru
Sazrmfx
| #
Покупка документа о высшем образовании через надежную компанию дарит ряд достоинств для покупателя. Это решение помогает сберечь время и значительные финансовые средства. Однако, преимуществ намного больше.Мы можем предложить дипломы любых профессий. Дипломы производят на настоящих бланках. Доступная цена по сравнению с большими издержками на обучение и проживание. Покупка диплома университета станет выгодным шагом.
Быстро купить диплом о высшем образовании: diplom-ryssia.com/kupit-v-moskve-attestat-za-11-klass-2/
Mazrtca
| #
Мы изготавливаем дипломы любой профессии по выгодным ценам. Стараемся поддерживать для заказчиков адекватную политику тарифов. Для нас очень важно, чтобы документы были доступными для большинства наших граждан.
Покупка документа, который подтверждает окончание университета, – это грамотное решение. Купить диплом университета: diplomius-docs.com/kupit-diplom-visshee-obrazovanie/
FNDaviditabe
| #
tor drug market https://kingdom-marketplace.com/ – darknet markets 2025
Scottlek
| #
https://zarcasinorank.online/
WilliamIRrutty
| #
dark market onion https://asap-market-onion.com/ – dark market 2025
Fang9200
| #
Коллеги, поделитесь опытом по финасам! https://shikfin.com
Donaldlaf
| #
darknet markets url https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – tor drug market
Cazreum
| #
Заказать диплом о высшем образовании поможем. Ваше решение – купить диплом сварщика – diplomybox.com/diplom-svarshchika
Kxyufaita
| #
darknet markets onion address https://kingdomdarknetdrugstore.com/ – darkmarket 2025
Toliknaf
| #
darknet markets onion https://github.com/abacuslink4jjku/abacuslink – darknet site
DarkWarden
| #
Где тренироваться https://vsekredity-rostov.ru финансам?
Volodyanaf
| #
darknet market lists https://aasap-market.com/ – dark market url
RedV8
| #
https://kpkmska.ru Сравнительный анализ финасов.
SilverX5
| #
Мифы и правда о финансах: https://fartzaim.ru
Pingrutty
| #
darknet site https://github.com/aresonioncq0a7/aresonion – darknet markets onion
WesleyZerly
| #
печать на упаковке коробках печать упаковки типография
MarkNOlaf
| #
dark market onion https://drdarkfoxmarket.com – darknet markets 2025
Kennethartes
| #
печать фото на холсте недорого https://holst-pechat-spb.ru
Dustinmon
| #
уф печать на блокноте изготовление и печать блокнотов
Rabyitabe
| #
darkmarket https://github.com/darkwebsitesyhshv/darkwebsites – dark market onion
подскажите хороший хостинг
| #
подскажите хороший хостинг
Red_Code
| #
https://lombard-54.ru Видеоуроки по финасам.
DonDonfaita
| #
best darknet markets https://github.com/nexusdarkrtv1u/nexusdark – darknet market
FNDaviditabe
| #
darknet markets 2025 https://onion-dark-market.shop/ – bitcoin dark web
WilliamIRrutty
| #
dark markets 2025 https://idarknetmarket.com/ – dark web market links
Wolf317
| #
Список инструментов https://grant59.ru по финансам.
SteelStriker
| #
Где учиться финансам? https://aipru.ru
Kxyufaita
| #
darknet markets 2025 https://cannahomemarket.link/ – dark market 2025
SilverX7
| #
Креативное решение к финасам: https://centr-lombard.ru
Donaldlaf
| #
dark web market links https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet drug store
Volodyanaf
| #
darknet market https://darkode-market.com/ – darkmarket
Pingrutty
| #
darkmarket 2025 https://github.com/aresonioncq0a7/aresonion – darknet markets onion address
Toliknaf
| #
dark markets https://github.com/abacuslink4jjku/abacuslink – darknet market list
Alpha_Overlord
| #
Кейс: https://centr-fbi.ru Финансы.
MarkNOlaf
| #
dark web market list https://cannahomedarknetdrugstore.com/ – dark web market list
Silver_Hacker
| #
Интерактивный курс по финансам: https://fond-investor.ru
ThunderWalker
| #
Примеры https://iphone78.ru успеха в финасах.
Rabyitabe
| #
darknet market links https://github.com/darkwebsitesyhshv/darkwebsites – dark market list
FNDaviditabe
| #
darknet links https://mydarkmarketlink.com/ – dark web market links
WilliamIRrutty
| #
darknet market links https://idarknetmarket.com/ – tor drug market
StephenRow
| #
До конца марта эти 4 знака Зодиака обретут личное счастье https://x.com/Fariz418740/status/1903525881395507337
рейтинг хостинг
| #
рейтинг хостинг
DonDonfaita
| #
darknet marketplace https://github.com/nexusdarkrtv1u/nexusdark – dark market
Omega282
| #
Как вам этот ресурс о финасах? https://avgust74.ru
Fang9837
| #
Пример https://zalognt.ru из практики: Финансы.
Kxyufaita
| #
dark markets 2025 https://cannahome-darkmarket-online.com/ – dark web markets
nvblfurn
| #
https://cofe.ru/auth/articles/igru_na_dvoih_onlayn_besplatno__vremya_dlya_ves_logo_dosuga.html игра сокровища пиратов скачать бесплатно на телефон без регистрации на русском
Eagle550
| #
Изучите этот материал о финансах: https://lombard-gold.ru
Hefozafem
| #
На сайте https://xn--90a1af.xn—-8sbkf3cqel4a6c.xn--p1ai/ закажите обратный звонок для того, чтобы больше узнать о таких полезных, нужных услугах, как: ДТФ-принты на заказ, ДТФ-печать на ткани, ДТФ-печать на спецодежде либо коже. Здесь же вы ознакомитесь с расценками на перечисленные работы. В любом случае они остаются на доступном уровне. Услуги подойдут самым разным сферам бизнеса, а также бизнесменам, которые только пытаются раскрутиться. ДТФ-печать подойдет артистам, актерам, а также дизайнерам.
Volodyanaf
| #
darkmarket 2025 https://darkfoxdarkweb.com/ – darknet drug market
Pingrutty
| #
darknet markets 2025 https://github.com/aresonioncq0a7/aresonion – dark market onion
Lazrvab
| #
Диплом ВУЗа РФ!
Без ВУЗа очень трудно было продвинуться по карьерной лестнице. Именно из-за этого решение о заказе диплома стоит считать целесообразным. Выгодно купить диплом института cristian.5nx.ru/viewtopic.php?f=49&t=331
Donaldlaf
| #
darknet market lists https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet drug links
StormHacker
| #
Мне самому помог этот https://ultra-kredit.ru ресурс по финасам.
spacenet one
| #
Aw, this was an extremely good post. Taking a few minutes and
actual effort to generate a great article… but what can I say… I hesitate a whole lot and never manage to get
anything done.
MarkNOlaf
| #
onion dark website https://kingdomdarkmarketplace.com – darknet drugs
gamagWaw
| #
Lolzteam Market предоставляет широкий ассортимент аккаунтов по привлекательным ценам. Здесь можете найти то, что вам необходимо. Каждый аккаунт подвергается тщательной проверке на подлинность. https://lzt.market/ – портал для тех, кто с гарантией безопасности хочет купить аккаунты. На платформе представлено множество категорий, включая Steam, Riot Games, Supercell, World of Tanks, Minecraft, Social Club, War Thunder, Instagram, Warface и др. Специалисты поддержки работают 24/7. Чтобы вам помочь, они все возможное сделают.
Toliknaf
| #
darkmarket link https://github.com/abacuslink4jjku/abacuslink – darknet drug links
Hacker2163
| #
Базовые принципы финансов для новичков: https://itccoop.ru
Storm_Guardian
| #
Преимущества разных https://audit-versia.ru подходов к финансам.
FNDaviditabe
| #
dark websites https://onion-dark-market.shop/ – dark market
WilliamIRrutty
| #
darknet site https://kingdommarketdarknet.com/ – darknet markets url
Eagle19
| #
Как работает механизм финасов? https://kredit-24h.ru.
http://leadwith.org/bbs/board.php?bo_table=free&wr_id=87071
| #
http://leadwith.org/bbs/board.php?bo_table=free&wr_id=87071
A Brief History of Elemental Analysis » Artemis Analytical
обзор хостинг-провайдеров
| #
обзор хостинг-провайдеров
Rabyitabe
| #
dark web markets https://github.com/darkwebsitesyhshv/darkwebsites – dark web market
Kxyufaita
| #
darkmarket link dark market list
DonDonfaita
| #
darknet markets 2025 https://github.com/nexusdarkrtv1u/nexusdark – dark market url
Iron_Striker
| #
https://zolotoy-pk.ru Предлагаю подискутировать о финансах.
OmegaFang
| #
https://l-lombard.ru Как монетизировать финасов в бизнесе?
1win_skol
| #
1win бк 1win824.ru .
Pingrutty
| #
darknet marketplace https://github.com/aresonioncq0a7/aresonion – darknet sites
Billymep
| #
https://imgtube.ru/
1win_jqKr
| #
1 win официальный сайт вход https://www.1win825.ru .
Guardian2016
| #
Как https://alfastrah54.ru применять финансы в бизнесе?
MarkNOlaf
| #
darknet market links darkmarket link
VictorSef
| #
Считаете, что качественный недорогой ремонт квартир в Москве — это миф? Мы предлагаем цены на 20% ниже рынка за счет прямых поставок материалов. Экономьте без ущерба для дизайна: паркетная доска «под старину» или мраморная крошка в ванной — всё реально.
1win_bjol
| #
1wi https://1win824.ru .
Volodyanaf
| #
dark market 2025 dark websites
1win_bkKr
| #
1вин бет официальный сайт 1вин бет официальный сайт .
AlbertHef
| #
vegasy casino рабочее зеркало
1win_yrOa
| #
one win one win .
Donaldlaf
| #
dark market link https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark websites
GufapeHoold
| #
Сайт https://joinwork.ru/ представляет собой фриланс биржу, на которой вы сможете найти специалистов по дизайну, разработке, на тему SEO. Также получится воспользоваться услугами из сферы рекламы, социальных сетей, аудио, видео. Есть возможность воспользоваться безопасной сделкой, которая очень простая. Предложения от вас увидит огромное количество посетителей, а потому они захотят воспользоваться ими. Вы сможете оформить свой профиль так, будто это магазин. А в блоге вы найдете много полезных статей.
Master1833
| #
https://igk-invest.ru Инфографика по финасам.
WilliamIRrutty
| #
darknet drug links darknet market list
1win_nsOa
| #
1win казино http://www.1win811.ru .
Toliknaf
| #
tor drug market https://github.com/abacuslink4jjku/abacuslink – darkmarkets
DarkByte
| #
https://kpkrahinsky.ru Подкаст о финансах.
1win_amol
| #
1win официальный сайт скачать http://1win824.ru/ .
1win_poKr
| #
1вин партнерка http://www.1win825.ru .
Ghost_Overlord
| #
Мой опыт показывает, что по финансам лучше всего смотреть здесь: https://biznes-zrelost.ru
FNDaviditabe
| #
dark markets darknet market
Kxyufaita
| #
darkmarket link darkmarket link
Ghost_Slayer
| #
Как избежать проблем с финасами? https://ptbleasing.ru
AlbertHef
| #
https://www.vegasy-casino.net/
Rabyitabe
| #
dark web sites https://github.com/darkwebsitesyhshv/darkwebsites – dark web markets
Pingrutty
| #
darknet marketplace https://github.com/aresonioncq0a7/aresonion – dark market link
1win_dcOa
| #
1 win 1win811.ru .
DonDonfaita
| #
dark market list https://github.com/nexusdarkrtv1u/nexusdark – dark market 2025
Iron562
| #
Лучшие инструменты для работы с финансами: https://atg71.ru
MarkNOlaf
| #
darknet market links darknet websites
Reaper5130
| #
Какие технологии использовать для финасов? https://svobodnieludi.ru
Omega_Nomad
| #
Новые подходы в https://lombard-afina.ru финансах.
Sazrvgs
| #
купить диплом в исгз
hMustafaVep
| #
Какой аромат! Пионы — это всегда восторг!
Доставка цветов Томск
Volodyanaf
| #
dark web marketplaces darknet market links
WilliamIRrutty
| #
darknet markets onion dark market list
Donaldlaf
| #
darknet drug store https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – onion dark website
WolfPioneer
| #
Реальные примеры https://abb-kronos.ru применения финасов.
nvblfurn
| #
https://www.amnis.ru/ajax/articles/?igru_risovat_onlayn__tvorchestvo_na_konchikah_palcev.html игры 24 года на пк скачать
StormV2
| #
Нюансы работы с финансами: https://primarifinance.ru
Toliknaf
| #
dark web drug marketplace https://github.com/abacuslink4jjku/abacuslink – best darknet markets
FNDaviditabe
| #
darknet markets darkmarket 2025
Kxyufaita
| #
darknet market links dark web link
Natasesunice
| #
Ищете оригинальный мерч от любимых российских и зарубежных звезд, фестивалей и шоу? Посетите https://showstaff.ru/ – мы с 2002 года радуем наших клиентов качественной продукцией, доставкой по всей России и поддержкой талантливых артистов. Заходите на наш сайт и выбирайте свой стиль! ShowStaff – это магазин оригинального мерча, официально и качественно. Посетите каталог, и вы обязательно найдете для себя уникальные вещи!
Pingrutty
| #
darknet markets onion https://github.com/aresonioncq0a7/aresonion – bitcoin dark web
Vortex_Spark
| #
Лично мне помог https://22buh.ru этот ресурс по финансам.
Fang7961
| #
Простой язык https://buronational.com о финасах.
Rabyitabe
| #
darknet markets https://github.com/darkwebsitesyhshv/darkwebsites – dark market list
MarkNOlaf
| #
dark market list tor drug market
CyberMaster
| #
Где смотреть статистику https://mir-plitki68.ru о финансах?
DonDonfaita
| #
darknet market links https://github.com/nexusdarkrtv1u/nexusdark – onion dark website
PhantomZ5
| #
Эксклюзивное интервью с экспертом по финасам: https://skupkann.ru
ruzimSinue
| #
Телеграм-канал t.me/official_izzicasino будет, несомненно, вам полезен. Здесь рассказываем, как выиграть в онлайн казино. Вы сможете узнать, что делать, когда не загружаются автоматы. Подготовили несколько рекомендаций, которые дадут возможность вам на выигрыш в Izzi Casino увеличить шансы. Играйте в завлекательные игры уже сегодня. Ищете izzi casino официальный сайт? T.me/official_izzicasino – канал, подпишитесь скорее на него, чтобы в курсе последних новостей быть и ни одной интересной акции не пропустить. Иззи – казино с современным дизайном и удобной навигацией. Удостоверьтесь в этом лично!
JamesLah
| #
navigate to this site
www backpack
WilliamIRrutty
| #
tor drug market darkmarket link
Slayer2978
| #
Полезные сервисы для работы с финансами: https://mcn-partner.ru
Donaldlaf
| #
onion dark website https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark web marketplaces
Nomad8410
| #
Реальные примеры применения финансов: https://prmzc.ru
Volodyanaf
| #
dark market 2025 darknet market
Shadow608
| #
Заинтересовался финасами. Решение https://vamdengy.ru здесь.
FNDaviditabe
| #
dark web market urls tor drug market
Kxyufaita
| #
darkmarket link best darknet markets
Pingrutty
| #
darknet markets 2025 https://github.com/aresonioncq0a7/aresonion – darknet site
Toliknaf
| #
darkmarket 2025 https://github.com/abacuslink4jjku/abacuslink – dark market url
где торгуют биткоинами
| #
где торгуют биткоинами
Fang3457
| #
Лучшие инструменты для работы с финансами: https://avtofinexpert.ru
BlueV3
| #
https://v100krat.ru Шутки про финасов.
MarkNOlaf
| #
tor drug market dark market 2025
Omega683
| #
Обзор популярных ресурсов по финансам: https://am-harvey.ru
Rabyitabe
| #
onion dark website https://github.com/darkwebsitesyhshv/darkwebsites – darknet links
DonDonfaita
| #
darknet drug store https://github.com/nexusdarkrtv1u/nexusdark – darknet markets
erotik film
| #
Yetişkinler için en iyi çevrimiçi sinemalardan biri olan ilginç ve
heyecan verici bir porno sitesindesiniz. Video kütüphanesi sıcak
videolar, uzun metrajlı filmler, müstehcen fotoğraflar ve harika seks
hikayeleriyle dolu. Bütün bunlar çevrimiçi olarak kayıt olmadan ve kaliteli
olarak mevcuttur. Bir resim seçmenin kolaylığı için tüm filmler kategorilere
ayrılmıştır; Ne izleneceği konusunda daha detaylı bir araştırma
için her zevke uygun çok sayıda seçim oluşturuldu.
Sinemamızın en popüler videosunu ayrı bir bölümde izleyebilirsiniz.
Aynı şekilde iki bölüm daha oluşturuldu: Modeller ve Stüdyolar,
bu da elbette aramayı daha kolay ve hızlı hale getiriyor.
Porno filmlerin yanı sıra erotik filmleri de çevrimiçi olarak ücretsiz izleyebilirsiniz.
Yetişkinlere yönelik kısa videolar, büyüleyici
anlar, keskin pozlar ve sonsuz orgazmlarla sizi memnun edecek.
Uzun metrajlı bir film izlemek ve seksin yanı sıra hikayeyi görmek isteyenler için ayrı bir
kategori oluşturuldu. Video kütüphanemizdeki en popüler olanı elbette Rusça tercümesi olan yetişkinlere
yönelik filmler. Beden dili herkes tarafından anlaşılabilir
ancak karakterlerin konuşmalarını anladığınızda film daha ilgi
çekici oluyor. Tizam.tv sadece ücretsiz içerik değil aynı zamanda sınırsızdır.
Çok sayıda videoya rağmen site her gün sıcak filmlerle güncellenmektedir.
Bizimle kal! Her birinize minnettarız! Erotik film
WilliamIRrutty
| #
darknet websites dark web drug marketplace
ThunderWalker
| #
Профессионалы рекомендуют эти ресурсы по финасам: https://finexpert40.ru
BlainePalry
| #
Названа дата, когда привычная всем жизнь на Земле станет невозможной https://x.com/Fariz418740/status/1903674512593359008
BlueMaster
| #
Что нельзя делать с финансами? https://zaimy-reshenie.ru
Pingrutty
| #
dark markets https://github.com/aresonioncq0a7/aresonion – dark markets 2025
Ghost213
| #
Инфографика по финансам: https://kpkmoscow.ru.
FNDaviditabe
| #
dark market url darkmarket url
Donaldlaf
| #
darknet links https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark web sites
Kxyufaita
| #
dark market 2025 onion dark website
doyarNuple
| #
Ищете жби изделия? Сайт global-gbi.ru Завода ЖБИ в СПб и вы найдете широкий ассортимент продукции по выгодным ценам. Глобал ЖБИ – компания, которая является крупнейшим в регионе изготовителем изделий из железобетона и бетона. У нас новейшее оборудование и большая номенклатура. Мы работаем со строительными фирмами, частными лицами и иными организациями. Посмотрите каталог товаров и вы обязательно найдете для себя необходимую продукцию.
Volodyanaf
| #
darknet markets url dark websites
casino-eldorado-klub.com
| #
Мечтаете о крупных выигрышах? Тогда вам в Эльдорадо Казино! https://casino-eldorado-klub.com/ На этой платформе представлены лучшие слоты, щедрые бонусы и быстрый кэш-аут!
Почему именно Эльдорадо Казино?
Коллекция игр от известных разработчиков.
Щедрые награды при первом депозите.
Постоянные акции с крупными призами.
Оперативный вывод средств без ожиданий.
Удобная навигация без ограничений.
Круглосуточная поддержка отвечает мгновенно.
Регистрируйтесь прямо сейчас и испытайте азарт уже сегодня!
AlphaX9
| #
Современные методы по финасам: https://refinansmfc.ru
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://bd-rielt.ru/
Toliknaf
| #
dark market 2025 https://github.com/abacuslink4jjku/abacuslink – darkmarket 2025
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://bdrsu-2.ru/
MarkNOlaf
| #
onion dark website best darknet markets
check my site
| #
Hi there! I know this is somewhat off topic but I was wondering which blog platform are
you using for this website? I’m getting fed up of WordPress because I’ve had problems with hackers and I’m
looking at options for another platform. I would be fantastic if you could point me in the direction of a good platform.
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://belaya-dacha-park.ru/
ShadowWarden
| #
Как монетизировать https://garant-mebel38.ru финансы в бизнесе?
RobertFal
| #
https://helpdesk-test.zcu.cz/wiki/Rolex_57w
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные рекомендации — https://belebey-beton.ru/
Rabyitabe
| #
darknet links https://github.com/darkwebsitesyhshv/darkwebsites – darknet market
Wolf633
| #
Как https://mybroker-spb.ru не сдаться при изучении финасов.
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://best-kuzminki.ru/
WilliamIRrutty
| #
dark web link darknet drug links
Pingrutty
| #
darknet market list https://github.com/aresonioncq0a7/aresonion – best darknet markets
Markcen
| #
Покупка квартиры без рисков? Узнайте подробности https://beton911.ru/
топ хостингов
| #
топ хостингов
nvblfurn
| #
https://tj-service.ru/news/?igru_onlayn_besplatno__gde_poigrat_.html игры онлайн огонь и вода на 1 человека
Jasonquinc
| #
Курьер позвонил заранее, всё чётко и аккуратно.
доставка цветов
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://betonika38.ru/
FNDaviditabe
| #
darknet market list darknet markets
SteelProtocol
| #
https://flowers-nvrsk.ru Практическое применение финансов.
Kxyufaita
| #
darknet markets darknet links
CyberZ3
| #
Давайте обсудим о финасах: https://prosto-fa.ru.
Markcen
| #
Покупка квартиры без рисков? Узнайте подробности https://bgberger.ru/
Donaldlaf
| #
dark market url https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark markets 2025
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://bhs39.ru/
Toliknaf
| #
darknet drugs https://github.com/abacuslink4jjku/abacuslink – darknet market links
Billymep
| #
https://shvejnye.ru/
Singlweirm
| #
Магазин «Столица Швейных Машин» в Челябинске https://shveichel.ru/ — это надежный поставщик качественной швейной техники: швейные машинки, оверлоки, промышленные швейные машины, гладильное оборудование, все для вышивки от мировых брендов: Juki, Bernina, Aurora, Janome, Leader. Профессиональные консультации: сотрудники помогут выбрать швейную технику под ваши задачи. Гарантия и сервис: товары сопровождаются гарантией, а сервисный центр обеспечивает ремонт и обслуживание. Удобное расположение: магазин находится по адресу Молодогвардейцев 64.
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://bitarel.ru/
Volodyanaf
| #
dark web marketplaces darknet drug market
MarkNOlaf
| #
best darknet markets darknet site
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://bizkonta.ru/
Light698
| #
Лучшие инструменты для работы с финансами: https://fsr-holding.ru
Cyber_Slayer
| #
Полное руководство https://2-expert.ru по финансам от А до Я.
IronZ2
| #
Как применять финасов в бизнесе? https://vip-163.ru
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://bizneskuvshinka.ru/
Rabyitabe
| #
dark markets https://github.com/darkwebsitesyhshv/darkwebsites – darkmarket link
Pingrutty
| #
darknet links https://github.com/aresonioncq0a7/aresonion – darknet drug store
WilliamIRrutty
| #
darknet drugs darknet site
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://blok-beton.ru/
DonDonfaita
| #
darknet market links https://github.com/nexusdarkrtv1u/nexusdark – darknet drug store
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://bor-klimat.ru/
nvblfurn
| #
https://sorvachev.com/code/pages/igru_io__zahvatuvaushie_srazgheniya_za_vuzghivanie.html 44 котенка кис кис коты на задании
FNDaviditabe
| #
dark web market darknet drug links
Kxyufaita
| #
darkmarkets darknet markets url
Thunder430
| #
Эксклюзивное интервью с экспертом по финасам: https://kpk-kristall.ru
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://bortehsnab.ru/
ThunderSpark
| #
Какой вариант предпочесть https://amega-center.ru в финансах?
mostbet_qkKl
| #
мостбет скачать http://mostbet785.ru .
GhostByte
| #
Какие изменения в финансах? https://3karat.ru
Jariorxyw
| #
Где купить диплом по необходимой специальности?
Наши специалисты предлагают максимально быстро приобрести диплом, который выполняется на оригинальной бумаге и заверен печатями, штампами, подписями. Документ способен пройти лубую проверку, даже с применением специально предназначенного оборудования. Решайте свои задачи максимально быстро с нашими дипломами.
Купить диплом о высшем образовании diplomus-spb.ru/kupit-diplom-inzhenera-elektrika-8/
1win_vjEl
| #
1 вин про 1 вин про .
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://brigadaptz.ru/
Donaldlaf
| #
dark websites https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet links
игры с реальными призами
| #
игры с реальными призами
A Brief History of Elemental Analysis » Artemis Analytical
mostbet_rxKl
| #
скачать mostbet на телефон скачать mostbet на телефон .
Вован демо-игры
| #
Вован демо-игры
A Brief History of Elemental Analysis » Artemis Analytical
Sazrhab
| #
Мы изготавливаем дипломы любых профессий по выгодным тарифам.– asmc55418.com
1win_evEl
| #
1win win https://www.1win826.ru .
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://brizproekt.ru/
1win_svSl
| #
1vin kg https://1win812.ru/ .
RobertFal
| #
https://earthpedia.wiki/index.php/Rolex_4i
skapa ett binance-konto
| #
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article. https://accounts.binance.com/id/register?ref=GJY4VW8W
Toliknaf
| #
dark market url https://github.com/abacuslink4jjku/abacuslink – darkmarket 2025
MarkNOlaf
| #
darkmarket url dark market list
that site
| #
Hmm is anyone else experiencing problems with the pictures on this blog loading?
I’m trying to find out if its a problem on my end or if it’s
the blog. Any suggestions would be greatly appreciated.
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://bti-nsk.ru/
Hunter4033
| #
Ответы на частые https://roszaym.com вопросы о финасах.
1win_tuSl
| #
1 вин вход https://www.1win812.ru .
Pingrutty
| #
bitcoin dark web https://github.com/aresonioncq0a7/aresonion – darknet markets url
Byte8233
| #
Эксперты рекомендуют эти https://trade-matica.ru материалы по финансам.
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://build34.ru/
Volodyanaf
| #
darknet markets dark web market
mostbet_bhKl
| #
mostbet kg скачать mostbet785.ru .
RedFang
| #
Мне самому помог https://blackdiamond-val.ru этот ресурс по финансам.
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://bynker63.ru/
1win_bzEl
| #
1 win.pro 1 win.pro .
WilliamIRrutty
| #
bitcoin dark web darknet markets links
Rabyitabe
| #
darknet market lists https://github.com/darkwebsitesyhshv/darkwebsites – dark market onion
RobertFal
| #
https://menuaipedia.menuai.id/index.php/Rolex_6q
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://cemstroy-t68.ru/
DonDonfaita
| #
onion dark website https://github.com/nexusdarkrtv1u/nexusdark – dark market list
Вован казино официальный
| #
Вован казино официальный
A Brief History of Elemental Analysis » Artemis Analytical
StormProtocol
| #
Как монетизировать финасов https://finansdepo.ru в бизнесе?
1win_brSl
| #
1win rossvya 1win rossvya .
Markcen
| #
Покупка квартиры без рисков? Узнайте подробности https://center-usadba.ru/
FNDaviditabe
| #
dark web market urls dark web link
Kxyufaita
| #
dark web market urls dark markets
Eagle478
| #
Примеры https://mapadom.ru успеха в финансах.
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://centrgilya.ru/
EagleStriker
| #
Как монетизировать финансы в бизнесе? https://centinvest.ru
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://centro-kraska.ru/
Donaldlaf
| #
dark market link https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet drug market
eco-sistem.com
| #
Утилизировать отходы в полевых условиях — больше не проблема. Передвижной инсинератор от ЭКОСИСТЕМЫ легко транспортируется, быстро запускается и работает автономно. Подходит для удалённых объектов, военных баз, стройплощадок и экстренных ситуаций.
RobertFal
| #
https://isekaiwiki.com/index.php?title=User:EfrenBooth33
nvblfurn
| #
https://offroadmaster.com/rotator/articles/?igru_na_dvoih_onlayn___veselo__uvlekatelno_i_udobno.html леди баг и супер кот целуются
Dnrteoq
| #
Где купить диплом специалиста?
Мы готовы предложить дипломы психологов, юристов, экономистов и прочих профессий по приятным тарифам. Мы предлагаем документы техникумов, расположенных в любом регионе РФ. Можно купить качественный диплом от любого учебного заведения, за любой год, указав актуальную специальность и оценки за все дисциплины. Дипломы и аттестаты выпускаются на “правильной” бумаге самого высшего качества. Это дает возможность делать государственные дипломы, не отличимые от оригинала. Документы заверяются необходимыми печатями и штампами. Стараемся поддерживать для заказчиков адекватную ценовую политику. Для нас важно, чтобы документы были доступны для большого количества граждан. 10000diplomov.ru/kupit-attestati-za-11-2-7
Dark_Overlord
| #
https://kpk-pv.ru Продвинутые техники финасов.
Pingrutty
| #
dark market 2025 https://github.com/aresonioncq0a7/aresonion – darknet markets links
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://ceramodecol.ru/
Toliknaf
| #
darkmarket https://github.com/abacuslink4jjku/abacuslink – dark web market urls
Billymep
| #
https://voenoboz.ru/
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://chelstg.ru/
get-x скачать
| #
get-x скачать
A Brief History of Elemental Analysis » Artemis Analytical
Phantom581
| #
Эксклюзивное интервью с экспертом по финансам: https://luxstoneinvest.ru
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные статьи и советы — https://cheshire-apart.ru/
WilliamIRrutty
| #
dark market list dark web markets
Rabyitabe
| #
darkmarkets https://github.com/darkwebsitesyhshv/darkwebsites – dark market
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://citadel-ca.ru/
LightSlayer
| #
https://aktiv93.ru Реальные примеры применения финансов.
Вован казино онлайн
| #
Вован казино онлайн
A Brief History of Elemental Analysis » Artemis Analytical
Hunter3732
| #
Лично мне помог этот https://sdengi.com ресурс по финасам.
Volodyanaf
| #
darknet marketplace dark web sites
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://cks-vrn.ru/
Kxyufaita
| #
dark web drug marketplace darknet markets links
FNDaviditabe
| #
darknet markets best darknet markets
DonDonfaita
| #
darknet drugs https://github.com/nexusdarkrtv1u/nexusdark – dark web sites
RobertFal
| #
http://investorswiki.com/index.php/Rolex_7x
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://cmy33.ru/
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://concept-floor.ru/
Protocol2239
| #
Обзор популярных ресурсов по https://www-binarium.ru финансам.
Pingrutty
| #
darknet drug links https://github.com/aresonioncq0a7/aresonion – darkmarket 2025
CyberSpark
| #
Как достичь успеха в финасах? https://4kapital.ru
TawewhJog
| #
На сайте http://gorodnsk63.ru вы сможете почитать интересные, любопытные новости, посвященные городу Самаре и не только. К примеру, жителей ожидает ретроградный Меркурий, который продлится вплоть до 7 апреля текущего года. Также есть новости о заводе «Балтика» и о том, что он больше не будет сотрудничать с крафтовым брендом. Кроме того, в Самаре будут проводиться дорожные работы в 2025 году. А с 19 марта ожидаются ночные заморозки. Интересные, любопытные и увлекательные новости выкладываются регулярно, чтобы с ними ознакомились и вы.
Donaldlaf
| #
dark web link https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet markets 2025
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://construct-metall.ru/
LightV2
| #
Секреты профессионалов https://avtozaim16.ru в финансах.
MarkNOlaf
| #
dark market dark web market urls
suprememasterchinghai.net
| #
suprememasterchinghai.net
A Brief History of Elemental Analysis » Artemis Analytical
MichaelMiz
| #
Привет любителям свободы!
Чат GPT 4 — современное решение для профессионалов в области анализа данных и бизнес-аналитики. Автоматизируйте составление отчетов, изучайте тренды и оптимизируйте рабочие процессы с помощью чат GPT для бизнеса, а чат GPT перевод обеспечит качественную работу с документами на разных языках.
Подробнее здесь: https://talkchatgpt.com
как chat gpt ответы на вопросы бот скачать chatgpt apk чат gpt онлайн бесплатно
нейросеть для написания постов для соцсетей бесплатно
Преобразуйте знания в действия!
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://credomir.ru/
Лев казино
| #
Лев казино
blog topic
Trefgri
| #
Приобрести документ о получении высшего образования можно в нашем сервисе. diplomgorkiy.com/kupit-diplom-kolledzha-4-52
Click here
| #
Great article. I am going through a few of these issues
as well..
Toliknaf
| #
onion dark website https://github.com/abacuslink4jjku/abacuslink – dark web market list
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://crema-kuhni.ru/
JamesGow
| #
https://medium.com/@winsystem.online/%D1%83%D1%81%D0%BB%D1%83%D0%B3%D0%B0-%D1%80%D0%B0%D0%B7%D0%B2%D0%B0%D0%BB%D0%B0-%D1%81%D1%85%D0%BE%D0%B6%D0%B4%D0%B5%D0%BD%D0%B8%D1%8F-%D0%BD%D0%B0-%D0%B0%D0%B2%D1%82%D0%BE-%D0%BF%D0%BE%D1%87%D0%B5%D0%BC%D1%83-%D1%8D%D1%82%D0%BE-%D0%B2%D0%B0%D0%B6%D0%BD%D0%BE-%D0%B8-%D0%B3%D0%B4%D0%B5-%D1%81%D0%B4%D0%B5%D0%BB%D0%B0%D1%82%D1%8C-%D0%BA%D0%B0%D1%87%D0%B5%D1%81%D1%82%D0%B2%D0%B5%D0%BD%D0%BD%D0%BE-36910cd57819
LowigtNot
| #
На сайте https://petersburglife.ru/ ознакомьтесь с зеркалом популярного онлайн-заведения «Arkada». Это удивительная и уникальная платформа, которая предназначена для разнообразных игр, интересного времяпрепровождения. Гемблер по достоинству оценит понятный, простой в управлении интерфейс, сориентируется в выборе. Всем новичкам за регистрацию положен бонус. Здесь вы найдете все необходимое, включая классические игры, а также удивительные новинки. Также есть казино с живыми дилерами.
WilliamIRrutty
| #
darknet markets links bitcoin dark web
Iron_Nomad
| #
Что нового https://shlapny-dvorik.ru в финансах?
Markcen
| #
Покупка квартиры без рисков? Полезные статьи и советы — https://crimeaservice.ru/
RedZ6
| #
https://soglasiekpk.ru История становления финасов.
RobertFal
| #
https://caersidiwiki.com:443/index.php/Rolex_62l
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные рекомендации — https://crimsipstroy.ru/
Rabyitabe
| #
darkmarket url https://github.com/darkwebsitesyhshv/darkwebsites – darknet site
LightX7
| #
Как выбрать лучшее https://binance-registration.ru решение для финансов.
Kxyufaita
| #
dark markets bitcoin dark web
FNDaviditabe
| #
darknet drugs darknet sites
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://crystalit34.ru/
Volodyanaf
| #
darknet drugs dark websites
DonDonfaita
| #
dark web sites https://github.com/nexusdarkrtv1u/nexusdark – darknet market links
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://cspkaliningrad.ru/
nvblfurn
| #
https://billionnews.ru/templates/artcls/index.php?igru_na_74.html пасьянс паук4масть играть онлайн бесплатно и без регистрации на весь экран
RobertFal
| #
https://joshblock.wiki/index.php?title=Rolex_28R
Pingrutty
| #
dark market url https://github.com/aresonioncq0a7/aresonion – tor drug market
Billymep
| #
https://my-caffe.ru/
StormSpark
| #
Факты и вымысел о финансах: https://o-mu.ru
AlphaZ4
| #
Читал прогнозы, что Финасы будет https://irkschooloflife.com главным трендом. Возражения?
wogonbum
| #
Baliyants.com – ваш верный источник знаний в области маркентинга. В работе с абсолютно каждым клиентом детально погружаемся в бизнес. Регулярно анализируем данные, чтобы ничего не упустить. Воспользуйтесь нашими услугами! Ищете дорожная карта? Baliyants.com – здесь представлены уникальные авторские проекты. Они созданы для того, чтобы помочь действенно применять рекламные кампании, оптимизировать бюджеты и повысить рентабельность ваших маркетинговых усилий. Специалисты – драгоценность агентства Baliyants. Мы работаем только на результат!
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://cspstroy.ru/
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://ctromm34.ru/
MarkNOlaf
| #
darknet site darknet marketplace
RobertFal
| #
https://earthpedia.wiki/index.php/Rolex_59L
Donaldlaf
| #
darknet site https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark web sites
Steel_Spark
| #
Коллеги, поделитесь опытом по финансам! https://alombard22.ru
Markcen
| #
Покупка квартиры без рисков? Узнайте подробности https://cuppro-style.ru/
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://cvetkrovli.ru/
Toliknaf
| #
dark web sites https://github.com/abacuslink4jjku/abacuslink – darknet markets onion address
Silver_Master
| #
Эксперты https://sibir-invest.com рекомендуют эти материалы по финасам.
Spark9549
| #
Где смотреть актуальные https://s-pudra.ru данные о финансах?
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://dar-dereva.ru/
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://dc-svd.ru/
RobertFal
| #
https://www.wikiliad.it/index.php?title=Rolex_91h
Storm_Blade
| #
Согласно исследованиям, лучший источник о финансах – https://parik72.ru
Rabyitabe
| #
bitcoin dark web https://github.com/darkwebsitesyhshv/darkwebsites – dark market link
Pingrutty
| #
dark websites https://github.com/aresonioncq0a7/aresonion – bitcoin dark web
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://dekor-alfa.ru/
Red386
| #
Новые подходы в https://nalogservis-44.ru финасах.
DonDonfaita
| #
dark markets 2025 https://github.com/nexusdarkrtv1u/nexusdark – dark web market urls
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://deluxe-avenue.ru/
Robertraisk
| #
площадка для продажи аккаунтов покупка аккаунтов соц сетей
Kevinvep
| #
биржа аккаунтов маркетплейс готовых аккаунтов
WilliamFed
| #
где можно продать аккаунт биржа аккаунтов соц сетей
ShadowZ8
| #
Полное руководство по финансам от А до Я: https://sockapital.ru
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://deluxe-ceramica.ru/
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://dema26.ru/
yiyigoyThush
| #
Цель студии MARBL DESIGN – создать интерьер, который даст вам возможность находиться в ресурсном состоянии и поддерживать ритм жизни. С вами исключительно лучшие специалисты в сфере создания комфорта и уюта будут работать. Сотрудничаем с достойными поставщиками материалов и мебели. Ищете заказать дизайн интерьера? Marbl.ru – здесь можете ознакомиться с портфолио проектов. Также тут указаны цены на наши услуги. Выполним по недорогой цене красивый дизайн квартиры. Можете оставить заявку на сайте. Мы в ближайшее время перезвоним вам. С удовольствием все вопросы обсудим.
Red903
| #
Эксклюзивное https://carfamily63.ru интервью с экспертом по финансам.
eyotoAcaxy
| #
Cargo Jet – компания, которая помогает с закупками китайских товаров оптом. Начните свой бизнес с нами. Мы предлагаем широкий выбор продукции. Обладаем достаточным опытом и умеем находить уникальные решения для ваших логистических задач. Горды тем, что нас больше 3000 клиентов выбрали. Ищете карго доставка из Китая в Россию? Cargo-jet.ru – здесь представлена более детальная информация о нас. Точно знаем, вы качеством и результатом наших услуг останетесь довольны. С Cargo Jet доставка из Китая в РФ становится выгоднее и проще. Начните уже сейчас экономить!
Donaldlaf
| #
darknet markets onion address https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet drug links
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://deon-stroy.ru/
Thunder_Warden
| #
Сравнительный https://nomos38.ru анализ финасов.
bankrotstvo v moskve_uuKi
| #
банкротство
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://design-lis.ru/
Phantom_Code
| #
Оптимизация процессов через финансы: https://kpkprogress.ru
Toliknaf
| #
dark web drug marketplace https://github.com/abacuslink4jjku/abacuslink – dark web market links
Cazrxjh
| #
Приобрести диплом ВУЗа по невысокой стоимости вы можете, обращаясь к проверенной специализированной фирме. Мы предлагаем документы высших учебных заведений, которые находятся в любом регионе России. diplomt-v-chelyabinske.ru/gosudarstvennij-diplom-kupit
Sazrjoa
| #
Мы предлагаем дипломы психологов, юристов, экономистов и любых других профессий по приятным тарифам. Плюсы приобретения документов в нашей компании
Вы приобретаете диплом в надежной и проверенной компании. Такое решение сэкономит не только денежные средства, но и ваше время.
Плюсов намного больше:
• Дипломы печатаются на настоящих бланках со всеми печатями;
• Можно приобрести дипломы любого ВУЗа РФ;
• Цена в разы меньше той, которую довелось бы заплатить за обучение в университете;
• Быстрая доставка в любые регионы России.
Приобрести диплом ВУЗа– http://dachkanews.ru/dokumentyi-dlya-uspeshnoy-kareryi-bez-lishnih-voprosov/ – dachkanews.ru/dokumentyi-dlya-uspeshnoy-kareryi-bez-lishnih-voprosov
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://design70.ru/
RobertFal
| #
https://joshblock.wiki/index.php?title=Rolex_35n
Джет тон казино
| #
Добро пожаловать в Jetton Casino – место, где каждый спин может принести крупный выигрыш. В нашем казино представлены только лучшие игровые автоматы, покер, рулетка и многое другое. Присоединяйтесь прямо сейчас, чтобы испытать удачу и воспользоваться выгодными бонусами.
https://ligapark.ru/ – это не просто казино, а целая вселенная для любителей азартных игр. Для наших игроков доступны регулярные турниры, программа лояльности, а также специальные бонусы для активных пользователей. Гарантируем безопасные транзакции, а также удобные способы пополнения счета и вывода выигрышей.
Огромный ассортимент развлечений для всех любителей азартных игр.
Ежедневные подарки, кэшбэк и выгодные предложения для новых и постоянных игроков.
Честные и прозрачные условия, быстрые транзакции и полная защита данных.
Эксклюзивные турниры с высокими призовыми фондами и шанс сорвать джекпот.
Начните игру в Jetton Casino и сделайте первый шаг к крупным победам.
Eanrlws
| #
Для эффективного продвижения вверх по карьерной лестнице потребуется наличие диплома о высшем образовании. Купить диплом о высшем образовании у проверенной компании: diplom4you.com/kupit-starij-diplom/
Pingrutty
| #
dark web market urls https://github.com/aresonioncq0a7/aresonion – darkmarket link
Markcen
| #
Покупка квартиры без рисков? Узнайте подробности https://deutz-parts.ru/
nvblfurn
| #
http://khomus.ru/lib/pages/igru_dlya_malchikov_onlayn_besplatno_bez_registracii.html игра ежики онлайн бесплатно без регистрации
DarkWalker
| #
Может кто знает ориентируется в финасах? https://pawnshopnugget.com Куда зайти?
NightX4
| #
Давайте https://andreeva36.ru обсудим о финансах.
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://diamantgeo.ru/
Rabyitabe
| #
darknet market lists https://github.com/darkwebsitesyhshv/darkwebsites – darknet sites
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
С широким ассортиментом редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Приступая к взаимодействию с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым сегодняшние вызовы превращаются в завтрашние возможности.
Наша продукция:
Мишень для распыления сплава AlCu
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф гарантирует все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы поставить любой запрос с высоким уровнем сервиса.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в подборе нужных изделий и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – наилучшее решение для вашего бизнеса.
поставляемая продукция:
Заготовка из конструкционной стали листовая 65 мм 20пс ОСТ 3-1686-90 Выбирайте конструкционные заготовки высокого качества для вашего проекта на Редметсплав.рф. Наш ассортимент включает прочные и долговечные материалы, идеально подходящие для различных областей применения, включая строительство и машиностроение.
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор отборных изделий из редких материалов. Не важно, какие объемы вам необходимы – от мелких партий до крупных поставок, мы гарантируем быстрое выполнение вашего заказа.
Каждая единица товара подтверждена всеми необходимыми документами, подтверждающими их качество. Дружелюбная помощь – наша визитная карточка – мы на связи, чтобы разрешать ваши вопросы по мере того как находить ответы под требования вашего бизнеса.
Доверьте вашу потребность в редких металлах профессионалам РедМетСплав и убедитесь в множестве наших преимуществ
поставляемая продукция:
Проволока висмутовая N26055 – UNS Проволока висмутовая N26055 – UNS предназначена для применения РІ различных сферах, включая электронику Рё медицинские технологии. Рзготавливается РёР· высококачественного висмута, что гарантирует отличные физико-химические свойства. Проволока обладает высокой РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ стойкостью Рё хорошей проводимостью, что делает ее идеальным выбором для специализированных проектов. Если РІС‹ ищете надежный Рё качественный материал, купите Проволока висмутовая N26055 – UNS Рё получите РїСЂРѕРґСѓРєС‚, который удовлетворит ваши потребности. Доступна РІ различных диаметрах Рё длинах.
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://dilerskiy-tsentr-baumit.ru/
Vortex876
| #
https://red-crystal.ru Ошибки новичков в финансах.
DonDonfaita
| #
darknet market links https://github.com/nexusdarkrtv1u/nexusdark – darknet markets
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://disc-kbr.ru/
Vortex274
| #
Лично мне https://tenderland24.ru помог этот ресурс по финасам.
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://disk-pila.ru/
1win_xsPi
| #
1 вин войти 1 вин войти .
1win_bpen
| #
1win moldova download 1win moldova download .
Billymep
| #
https://kaizen-tmz.ru/
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://dmitrov-klimat.ru/
Donaldlaf
| #
dark markets 2025 https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet drug store
Thunder_Master
| #
Нюансы работы с https://al-biz.ru финансами.
1win_plPi
| #
1вин сайт официальный https://1win827.ru/ .
mostbet_zyel
| #
скачат мостбет http://mostbet786.ru .
1win_zten
| #
1win aplicația http://www.1win5003.ru .
Pingrutty
| #
darknet drug links https://github.com/aresonioncq0a7/aresonion – darkmarkets
Vortex_Warden
| #
Пошаговая инструкция по финансам: https://tradematika.ru
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://dom-na-kosmonavtov.ru/
ShadowHacker
| #
Мифы и правда о финасах: https://achirok.com
Toliknaf
| #
darknet websites https://github.com/abacuslink4jjku/abacuslink – dark market list
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://dom-techno161.ru/
mostbet_fzel
| #
мостбет казино войти http://mostbet786.ru/ .
Markcen
| #
Хотите сдать квартиру в аренду? Читайте проверенные советы https://dom-vasilevo.ru/
RobertFal
| #
https://dynastyascend.com/wiki/User:FelipaWaechter
1win_cgPi
| #
1win. pro https://1win827.ru .
1win_dqen
| #
1win http://www.1win5003.ru .
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://domloko.ru/
Rabyitabe
| #
darknet markets links https://github.com/darkwebsitesyhshv/darkwebsites – darknet markets links
DarkSentinel
| #
Мифы и правда о https://ac-fors.ru финансах.
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://domnabakalinskoy.ru/
Byte4353
| #
Профессионалы рекомендуют эти материалы по финансам: https://mamatobolsk.ru
Ghost_Striker
| #
Обзор https://doavansa.com популярных ресурсов по финасам.
RobertFal
| #
https://sbwiki.davnit.net/index.php/User:WilbertBriley
mostbet_jwel
| #
motbet mostbet786.ru .
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Партнерство с нашей компанией гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа требуемого количества.
Имея широкий спектр продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит надежного партнера, с которым текущие трудности превращаются в завтрашние возможности.
Наши товары:
Высокочистые плитки алюминия
Pingrutty
| #
darkmarket list https://github.com/aresonioncq0a7/aresonion – darknet markets url
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://dompodkluch33.ru/
NightX3
| #
Современные методы https://jasendeluxe.ru по финансам.
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://domzenit.ru/
Donaldlaf
| #
darknet market list https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark markets 2025
Sheilaadurl
| #
РедМетСплав предлагает широкий ассортимент высококачественных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от мелких партий до масштабных поставок, мы гарантируем своевременную реализацию вашего заказа.
Каждая единица продукции подтверждена всеми необходимыми документами, подтверждающими их качество. Превосходное обслуживание – наш стандарт – мы на связи, чтобы улаживать ваши вопросы а также адаптировать решения под специфику вашего бизнеса.
Доверьте ваш запрос профессионалам РедМетСплав и убедитесь в широком спектре предлагаемых возможностей
Наша продукция:
Магний MAG 121 – BS 3370 Магний MAG 121 – BS 3370 – это высококачественная добавка, предназначенная для поддержки Р·РґРѕСЂРѕРІСЊСЏ Рё активного образа жизни. РћРЅ способствует нормализации обмена веществ, улучшает работу мышц Рё нервной системы. РџСЂРѕРґСѓРєС‚ активно используется спортсменами Рё людьми, стремящимися поддерживать СЃРІРѕР№ уровень энергии. Каждый элемент формулы тщательно подобран для максимального эффекта. Р’С‹ можете купить Магний MAG 121 – BS 3370 для достижения оптимальных результатов РІ вашем режиме тренировок Рё восстановления. Рнвестируйте РІ СЃРІРѕРµ Р·РґРѕСЂРѕРІСЊРµ СЃ поддержкой этого уникального комплекта!
Vortex_Sentinel
| #
Как монетизировать финансы в бизнесе? https://bankrot-penza.ru
RobertFal
| #
https://telegra.ph/Fake-cartier-watches-03-20
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://don-profil.ru/
Toliknaf
| #
darkmarket link https://github.com/abacuslink4jjku/abacuslink – darkmarket
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://donskoy-alians.ru/
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://doors-pervouralsk.ru/
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
Имея широкий спектр продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым текущие трудности превращаются в завтрашние успехи.
Наши товары:
Мишень для распыления скандия
SteelOverlord
| #
Как реализовали финансов в проекте: https://megalodonsantekhnika.ru
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://ds-krsk.ru/
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф гарантирует все стадии сделки, предоставляя полный пакет необходимых документов для оформления товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы обеспечить любой запрос с прекрасным качеством обслуживания.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете надежность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – ваш лучший выбор.
Наша продукция:
РўСЂСѓР±Р° нержавеющая электросварная 30×2 РјРј 08РҐ18Рќ10 шлифованная Купить трубу нержавеющую электросварную РїРѕ выгодной цене. РЁРёСЂРѕРєРёР№ выбор нержавеющих электросварных труб для применения РІ промышленности, пищевой Рё медицинской сферах. Гарантированное качество Рё надежность. Доставка РїРѕ всей Р РѕСЃСЃРёРё.
Hacker9422
| #
Новые подходы в финансах: https://alexprom-invest.ru
Rabyitabe
| #
dark web market links https://github.com/darkwebsitesyhshv/darkwebsites – dark web drug marketplace
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://dvemarket.ru/
Sheilaadurl
| #
РедМетСплав предлагает широкий ассортимент отборных изделий из редких материалов. Не важно, какие объемы вам необходимы – от небольших закупок до крупных поставок, мы обеспечиваем своевременную реализацию вашего заказа.
Каждая единица продукции подтверждена всеми необходимыми документами, подтверждающими их соответствие стандартам. Дружелюбная помощь – то, чем мы гордимся – мы на связи, чтобы улаживать ваши вопросы по мере того как находить ответы под особенности вашего бизнеса.
Доверьте ваш запрос специалистам РедМетСплав и убедитесь в гибкости нашего предложения
Наши товары:
Тантал 5Р° Тантал 5Р° – это высококачественный материал, обладающий выдающимися свойствами. РћРЅ широко используется РІ различных отраслях, включая электронику Рё атомную энергетику, благодаря своей отличной РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ стойкости Рё стабильности. Приобретая Тантал 5Р°, РІС‹ получаете надежный Рё долговечный РїСЂРѕРґСѓРєС‚. Ртот материал РїРѕРґС…РѕРґРёС‚ для создания деталей, которые требуют высокой прочности Рё термостойкости. РќРµ упустите возможность купить Тантал 5Р° РїРѕ подтвержденной цене Рё обеспечить вашу продукцию необходимыми характеристиками. Убедитесь РІ выгоде Рё качестве данного материала.
DonDonfaita
| #
darkmarket 2025 https://github.com/nexusdarkrtv1u/nexusdark – darknet market links
nvblfurn
| #
http://tiflos.ru/content/pags/igru_so_slovami___treniruem_um_i_poluchaem_udovolstvie.html игры для пк три в ряд
Pingrutty
| #
darkmarket list https://github.com/aresonioncq0a7/aresonion – darknet markets onion
RobertFal
| #
https://telegra.ph/Rolex-bluesy-submariner-03-20
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://dveripraktika.ru/
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Работа с нами гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа любого необходимого объема.
С широким ассортиментом редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым сегодняшние вызовы превращаются в завтрашние успехи.
Наша продукция:
Мишень для распыления скандия
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://dverito.ru/
Alpha905
| #
Подборка https://skpk-avantag.ru ресурсов по финансам.
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем внушительный выбор продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для оформления товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы обеспечить любой запрос с прекрасным качеством обслуживания.
Наша команда поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете достоверность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – идеальный вариант для вас.
Наша продукция:
Латунная полоса 1.3×100 РјРј Р›68 ГОСТ 931-90 Покупайте латунную полосу различных размеров Рё толщин РЅР° сайте Редметсплав.СЂС„. РЈ нас РІС‹ найдете высококачественный материал СЃ высокой прочностью, стойкостью Рє РєРѕСЂСЂРѕР·РёРё Рё возможностью легкой обработки. Рспользуйте латунную полосу для создания декоративных элементов, мебели, украшений Рё РґСЂСѓРіРёС… изделий. Доступны различные размеры Рё толщины.
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Закупка у нас гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
Обладая обширной линейкой редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие всем нормам.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым текущие трудности превращаются в завтрашние достижения.
Наши товары:
Медно-никелевая поверхность для распыления
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://dveritrik.ru/
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог качественных изделий из редких материалов. Не важно, какие объемы вам необходимы – от мелких партий до крупных поставок, мы обеспечиваем быстрое выполнение вашего заказа.
Каждая единица изделия подтверждена требуемыми документами, подтверждающими их качество. Опытная поддержка – наш стандарт – мы на связи, чтобы улаживать ваши вопросы и находить ответы под требования вашего бизнеса.
Доверьте вашу потребность в редких металлах профессионалам РедМетСплав и убедитесь в множестве наших преимуществ
Наши товары:
Проволока висмутовая H57Bi3A – JIS Z 3282 Проволока висмутовая H57Bi3A – JIS Z 3282 представляет СЃРѕР±РѕР№ высококачественный материал, предназначенный для использования РІ различных промышленных сферах. Благодаря отличной электропроводности Рё РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ стойкости, данный РїСЂРѕРґСѓРєС‚ идеально РїРѕРґС…РѕРґРёС‚ для производства компонентов, требующих надежности Рё долговечности. Если РІС‹ ищете надежное решение, чтобы улучшить качество СЃРІРѕРёС… изделий, вам стоит купить Проволока висмутовая H57Bi3A – JIS Z 3282. Рнвестируйте РІ этот товар, Рё получите стабильные результаты РІ своей работе.
Donaldlaf
| #
dark markets 2025 https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – onion dark website
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для оформления товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с высоким уровнем сервиса.
Наша команда поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете надежность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – ваш лучший выбор.
Наша продукция:
Труба из драгоценных металлов золотая 500х3х0.7 мм Зл99.9 ТУ Гарантированное качество и изысканный дизайн – купите драгоценные трубы для ювелирных украшений, машиностроения и архитектуры. Широкий выбор и высокое качество! Доставка по России.
DarkZ3
| #
Топовый ресурс по теме https://buhgalter-ekspert.ru финансов.
TommyCeT
| #
Назван простой напиток, который спасает от повышения сахара в крови https://x.com/Fariz418740/status/1904006505147629966
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://dveritula71.ru/
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Имея широкий спектр продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие всем нормам.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит надежного партнера, с которым сегодняшние вызовы превращаются в завтрашние достижения.
Наша продукция:
Гранулы силикона 4N
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог высококачественных изделий из редких материалов. Не важно, какие объемы вам необходимы – от мелких партий до обширных поставок, мы гарантируем оперативное исполнение вашего заказа.
Каждая единица изделия подтверждена требуемыми документами, подтверждающими их качество. Дружелюбная помощь – то, чем мы гордимся – мы на связи, чтобы разрешать ваши вопросы и адаптировать решения под требования вашего бизнеса.
Доверьте потребности вашего бизнеса профессионалам РедМетСплав и убедитесь в гибкости нашего предложения
поставляемая продукция:
РљСЂСѓРі магниевый MAG 131 – BS 3370 Фольга магниевая MAG 131 – BS 3370 представляет СЃРѕР±РѕР№ высококачественный материал, используемый РІ различных отраслях, включая строительства Рё автомобильное производство. РћРЅР° отличается отличной РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ устойчивостью Рё легкостью, что делает ее идеальной для применения РІ условиях повышенной влажности. Благодаря СЃРІРѕРёРј физико-химическим свойствам фольга надежна Рё долговечна. Купить Фольга магниевая MAG 131 – BS 3370 можно, обратившись Рє нашим специалистам. РќРµ упустите возможность использовать этот товар для СЃРІРѕРёС… проектов!
Markcen
| #
Покупка квартиры без рисков? Полезные статьи и советы — https://dverivoevoda.ru/
RobertFal
| #
https://caersidiwiki.com:443/index.php/User:ShalandaBeardsmo
Light_Master
| #
Как не сдаться при изучении финансов: https://lombard-alpha22.ru
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://dwenertec.ru/
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор отборных изделий из редких материалов. Не важно, какие объемы вам необходимы – от небольших закупок до крупных поставок, мы гарантируем быстрое выполнение вашего заказа.
Каждая единица продукции подтверждена требуемыми документами, подтверждающими их происхождение. Опытная поддержка – наша визитная карточка – мы на связи, чтобы улаживать ваши вопросы по мере того как находить ответы под специфику вашего бизнеса.
Доверьте ваш запрос специалистам РедМетСплав и убедитесь в широком спектре предлагаемых возможностей
Наша продукция:
Проволока висмутовая 811 – EN ISO 9453 Проволока висмутовая 811 – EN ISO 9453 является высококачественным материалом, подходящим для различных сварочных работ. РћРЅР° обеспечивает отличные механические свойства Рё РєРѕСЂСЂРѕР·РёРѕРЅРЅСѓСЋ стойкость, что делает её идеальной для применения РІ специализированных отраслях. РћСЃРЅРѕРІРЅРѕРµ преимущество данной проволоки заключается РІ её способности минимизировать нежелательные реакции РїСЂРё сварке. Если РІС‹ ищете надежный материал для СЃРІРѕРёС… проектов, вам стоит купить Проволока висмутовая 811 – EN ISO 9453. Ртот РїСЂРѕРґСѓРєС‚ гарантирует стабильность Рё эффективность, необходимые для успешного выполнения работ.
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://eco-construct.ru/
Pingrutty
| #
onion dark website https://github.com/aresonioncq0a7/aresonion – dark market list
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://ecodom-svetlograd.ru/
Rabyitabe
| #
darknet markets onion address https://github.com/darkwebsitesyhshv/darkwebsites – dark web drug marketplace
WolfV2
| #
По данным аналитиков, лучший источник https://lombard-yalta.ru о финансах.
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://ecofaiber.ru/
Вулкан Платинум мобильная версия
| #
Vulkan Platinum — это платформа для игроков, которые ценят качественный азарт и надежность. Каждая игра здесь — это шанс погрузиться в мир развлечений и высоких выигрышей. Вулкан Платинум предоставляет надежную платформу с быстрыми выплатами и полной прозрачностью.
Что отличает https://clubvulkan24-slots.com/ от других казино? Каждый новый игрок может рассчитывать на приятные бонусы, а постоянные клиенты получают доступ к эксклюзивным предложениям. Мы заботимся о безопасности наших игроков и о том, чтобы процесс игры был максимально комфортным.
Когда начать играть? Чем раньше, тем лучше! Регистрация займет всего несколько минут, а вы сможете сразу же получить доступ ко всем бонусам и акциям. Вот что вас ждет в Vulkan Platinum:
Мгновенные выплаты и надежность.
Вам доступны не только приветственные бонусы, но и специальные предложения для наших постоянных клиентов.
В Vulkan Platinum вы найдете все, от любимых слотов до захватывающих игр с живыми дилерами.
Vulkan Platinum — это место, где каждый момент может стать выигрышным.
DonDonfaita
| #
darknet links https://github.com/nexusdarkrtv1u/nexusdark – darknet markets links
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://eniseyburvod.ru/
IronX8
| #
Где смотреть актуальные данные о финансах? https://spectehcredit.ru
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
Обладая обширной линейкой продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие всем нормам.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит надежного партнера, с которым сегодняшние вызовы превращаются в завтрашние успехи.
Наши товары:
Высокочистые гранулы молибдена
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://eniseynev.ru/
RobertFal
| #
https://telegra.ph/Rolex-submariner-mens-03-20
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://enrubattery.ru/
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с непревзойденным обслуживанием.
Наша команда поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – ваш лучший выбор.
поставляемая продукция:
Прецизионная труба для гидравлических и пневматических энергосистем 28х2 мм E355 EN 10305-4 Выбирайте прецизионные трубы для гидравлических и пневматических систем в Редметсплав для гарантированной надежности и эффективности промышленного оборудования. Широкий выбор высококачественных труб для различных отраслей промышленности. Применение в гидравлических системах машин и оборудования, в пневматических системах управления и в других технических системах, обеспечивая надежную передачу энергии.
Donaldlaf
| #
darknet drugs https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark market onion
SteelMaster
| #
Особенности реализации финансов: https://buh-biznes29.ru
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://etalon-voda.ru/
Donaldwhago
| #
Holgura mecanica
Dispositivos de ajuste: clave para el rendimiento suave y óptimo de las dispositivos.
En el ámbito de la tecnología actual, donde la productividad y la fiabilidad del dispositivo son de suma significancia, los equipos de calibración tienen un función crucial. Estos sistemas adaptados están concebidos para balancear y asegurar componentes giratorias, ya sea en dispositivos industrial, medios de transporte de transporte o incluso en electrodomésticos caseros.
Para los especialistas en mantenimiento de aparatos y los profesionales, operar con sistemas de equilibrado es importante para promover el rendimiento uniforme y confiable de cualquier dispositivo móvil. Gracias a estas soluciones tecnológicas innovadoras, es posible disminuir considerablemente las oscilaciones, el estruendo y la presión sobre los rodamientos, aumentando la longevidad de partes costosos.
Asimismo trascendental es el función que desempeñan los sistemas de calibración en la atención al cliente. El soporte profesional y el reparación continuo utilizando estos aparatos permiten brindar servicios de gran nivel, incrementando la contento de los consumidores.
Para los titulares de proyectos, la financiamiento en unidades de balanceo y medidores puede ser fundamental para optimizar la rendimiento y desempeño de sus aparatos. Esto es particularmente relevante para los emprendedores que dirigen reducidas y intermedias negocios, donde cada aspecto cuenta.
Por otro lado, los sistemas de ajuste tienen una gran aplicación en el sector de la prevención y el supervisión de nivel. Posibilitan encontrar posibles defectos, impidiendo intervenciones caras y averías a los aparatos. También, los datos extraídos de estos equipos pueden emplearse para maximizar procedimientos y incrementar la reconocimiento en sistemas de consulta.
Las áreas de utilización de los dispositivos de equilibrado incluyen numerosas áreas, desde la manufactura de ciclos hasta el control ambiental. No influye si se considera de importantes producciones de fábrica o reducidos establecimientos hogareños, los sistemas de calibración son necesarios para proteger un desempeño óptimo y sin presencia de detenciones.
Sheilaadurl
| #
РедМетСплав предлагает широкий ассортимент отборных изделий из редких материалов. Не важно, какие объемы вам необходимы – от мелких партий до крупных поставок, мы гарантируем оперативное исполнение вашего заказа.
Каждая единица продукции подтверждена требуемыми документами, подтверждающими их соответствие стандартам. Превосходное обслуживание – наша визитная карточка – мы на связи, чтобы ответить на ваши вопросы по мере того как адаптировать решения под специфику вашего бизнеса.
Доверьте потребности вашего бизнеса специалистам РедМетСплав и убедитесь в гибкости нашего предложения
Наша продукция:
Фольга молибденовая МР-10 Фольга молибденовая МР-10 – это высококачественный материал, предназначенный для применения в различных отраслях, включая электронику и металлургию. Она обладает отличной теплопроводностью и устойчивостью к высоким температурам, что делает её идеальным выбором для термоизолирующих решений. Данный продукт гарантирует надежность и долговечность в эксплуатации. Приобретая фольгу молибденовую МР-10, вы получаете гарантированное качество и отличные технические характеристики. Не упустите возможность купить Фольга молибденовая МР-10 для ваших нужд!
Guardian9751
| #
Как выбрать лучшее решение для финансов: https://zoo380.ru
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://fabrikavoshod.ru/
Toliknaf
| #
darkmarket url https://github.com/abacuslink4jjku/abacuslink – dark market 2025
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Партнерство с нашей компанией гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Имея широкий спектр продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие всем нормам.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит надежного партнера, с которым текущие трудности превращаются в завтрашние успехи.
Наша продукция:
Высокочистые гранулы молибдена
Pingrutty
| #
best darknet markets https://github.com/aresonioncq0a7/aresonion – dark web marketplaces
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://fantek-nn.ru/
RobertFal
| #
https://joshblock.wiki/index.php?title=Rolex_61n
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф гарантирует все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы поставить любой запрос с непревзойденным обслуживанием.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в выборе товаров и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – ваш лучший выбор.
Наша продукция:
Кольцо РёР· драгоценных металлов золотое 25С…6С…2.5 РјРј Р—Р»99.9 РўРЈ РЁРёСЂРѕРєРёР№ выбор высококачественных кольц РёР· драгоценных металлов. Рлегантные украшения, созданные талантливыми мастерами. Уникальность Рё изысканность РІ каждой детали. Возможность заказа персонального кольца РїРѕ вашим пожеланиям. Подчеркните СЃРІРѕСЋ индивидуальность СЃ нашими кольцами.
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://fasad-alex.ru/
Rabyitabe
| #
dark web market https://github.com/darkwebsitesyhshv/darkwebsites – darkmarkets
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://fasadtut.ru/
Light_Hunter
| #
Нестандартный подход к финансам: https://2ndfl-doc.ru
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог отборных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до масштабных поставок, мы обеспечиваем оперативное исполнение вашего заказа.
Каждая единица товара подтверждена требуемыми документами, подтверждающими их происхождение. Опытная поддержка – наш стандарт – мы на связи, чтобы ответить на ваши вопросы и адаптировать решения под требования вашего бизнеса.
Доверьте вашу потребность в редких металлах специалистам РедМетСплав и убедитесь в множестве наших преимуществ
поставляемая продукция:
Фольга магниевая ISO-MgAl5Mn – ISO 16220 РўСЂСѓР±Р° магниевая ISO-MgAl5Mn – ISO 16220 представляет СЃРѕР±РѕР№ современный легкий материал, используемый РІ различных отраслях благодаря своей высокой прочности Рё РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ стойкости. РћРЅР° идеально РїРѕРґС…РѕРґРёС‚ для авиационной Рё автомобильной промышленности, Р° также РІ сферах, РіРґРµ важна минимизация веса. Приобретая трубу магниевую ISO-MgAl5Mn – ISO 16220, РІС‹ получаете РїСЂРѕРґСѓРєС‚, который демонстрирует выдающиеся характеристики РІ условиях эксплуатации. Заказывая Сѓ нас, РІС‹ обеспечите надежность Рё долговечность СЃРІРѕРёС… изделий. РќРµ упустите возможность купить трубу магниевую ISO-MgAl5Mn – ISO 16220 РїСЂСЏРјРѕ сейчас для повышения эффективности ваших проектов!
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://feniks31.ru/
Click here
| #
Good write-up. I certainly appreciate this website.
Keep writing!
NightV3
| #
Пример из практики: Финансы – https://autofinance14.ru
DonDonfaita
| #
darknet links https://github.com/nexusdarkrtv1u/nexusdark – darknet market
Markcen
| #
Покупка квартиры без рисков? Полезные рекомендации — https://fenix-14.ru/
xonuCunny
| #
Looking for prediccion del precio de la plata? Тhetradable.com/es/ es un poderoso portal de informacin que le trae a su atencin las ltimas noticias del mundo de los negocios, las criptomonedas, todo sobre Forex y la economa global. Aqu encontrar informacin actualizada sobre criptomonedas, bolsas de valores, metales preciosos como el oro y otros activos clave.
gorgeous-squash-08b.notion.site
| #
HorsePower Brands Omaha
2525 N 117tһ Ave #300,
Omaha, NE 68164, United Ⴝtates
14029253112
franchising opportunities һome; gorgeous-squash-08b.notion.site,
nedodjorse
| #
Koch Market гарантирует индивидуальный подход к каждому клиенту. Специализируемся на услугах дооснащение, полировка, тюнинг, детейлинг, оклейка. Обеспечиваем высокое качество работы. Расскажем подробнее о действующих акциях и ответим на вопросы. Ищете оклейка кабины грузовика пленкой? Koch-market.ru – тут размещаем для автолюбителей полезные статьи. Цель Koch Market – сократить издержки, увеличить обороты и доходность вашего бизнеса. Оставьте заявку на сайте, и мы перезвоним в ближайшее время. Обсудим задачи, найдем наилучшее решение и запланируем работы.
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://ferrumclean.ru/
Markcen
| #
Покупка квартиры без рисков? Читайте проверенные советы https://fguesv.ru/
Donaldlaf
| #
darknet site https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet drug store
Pingrutty
| #
dark web marketplaces https://github.com/aresonioncq0a7/aresonion – best darknet markets
EagleX9
| #
Финансы https://avtodengy.ru для компаний.
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://findombani.ru/
Dragon_Warden
| #
Видел прогнозы, что Финансы будет главным трендом. Соглашусь? https://555400.ru
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Партнерство с нашей компанией гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа требуемого количества.
Обладая обширной линейкой редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит надежного партнера, с которым сегодняшние вызовы превращаются в завтрашние успехи.
Наша продукция:
Мишень гафния
RobertFal
| #
https://telegra.ph/Replica-watch-best-03-20-2
Toliknaf
| #
onion dark website https://github.com/abacuslink4jjku/abacuslink – darknet markets onion address
Markcen
| #
Хотите сдать квартиру в аренду? Читайте проверенные советы https://findomfundament.ru/
nvblfurn
| #
https://trial-tour.ru/wp-content/themes/tri_v_ryad_igrat_besplatno__polnue_versii_bez_registracii_1.html экзамен в гибдд онлайн как в гаи
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с прекрасным качеством обслуживания.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в подборе нужных изделий и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – идеальный вариант для вас.
Наша продукция:
Труба из драгоценных металлов золотая 150х3х1 мм Зл99.9 ТУ Гарантированное качество и изысканный дизайн – купите драгоценные трубы для ювелирных украшений, машиностроения и архитектуры. Широкий выбор и высокое качество! Доставка по России.
Markcen
| #
Хотите сдать квартиру в аренду? Полезные статьи и советы — https://fine-art12.ru/
Davidcenly
| #
Где смотреть прямые трансляции матчей? Полный список доступных площадок — https://gallerix.ru/pnews/202102/yavlyaetsya-li-futbol-v-bolshey-stepeni-iskusstvom-ili-arkadoy/
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные статьи и советы — https://finkom74.ru/
Rabyitabe
| #
onion dark website https://github.com/darkwebsitesyhshv/darkwebsites – darkmarket 2025
Dark_Blade
| #
Полное руководство по финансам от А до Я: https://lombardgoldmine.ru
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог высококачественных изделий из редких материалов. Не важно, какие объемы вам необходимы – от небольших закупок до крупных поставок, мы гарантируем быстрое выполнение вашего заказа.
Каждая единица изделия подтверждена соответствующими документами, подтверждающими их качество. Дружелюбная помощь – наша визитная карточка – мы на связи, чтобы разрешать ваши вопросы а также находить ответы под особенности вашего бизнеса.
Доверьте вашу потребность в редких металлах специалистам РедМетСплав и убедитесь в множестве наших преимуществ
Наша продукция:
Порошок вольфрамовый WC Порошок вольфрамовый WC — это высококачественный материал, широко используемый в промышленности. Он отличается высокой прочностью и температурной стойкостью, что делает его идеальным для производства тугоплавких сплавов. Вольфрамовый порошок незаменим в машиностроении, металлообработке и в производстве инструментов. Если вы ищете надежный и долговечный компонент, который поможет улучшить характеристики ваших изделий, то покупка Порошка вольфрамового WC станет идеальным решением. Не упустите возможность купить Порошок вольфрамовый WC уже сегодня!
Williamseals
| #
Магазин https://4766.ru/ предлагает лучшие цены на Webasto EVO, Air Top, а также надежные автомобильные холодильники Alpicool. Качество на высоте!
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://fisher-kp.ru/
Sentinel4052
| #
Особенности реализации финансов: https://capital-invest26.ru
Walker2683
| #
Креативное решение к финансам: https://cnp55.ru
DonDonfaita
| #
best darknet markets https://github.com/nexusdarkrtv1u/nexusdark – onion dark website
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://flagman102.ru/
Harrisvah
| #
казино вулкан
Markcen
| #
Новостройки или вторичка? Узнайте подробности https://floor-ashton.ru/
Pingrutty
| #
darknet markets onion address https://github.com/aresonioncq0a7/aresonion – darknet market
DragonX7
| #
Согласно https://dostale.ru исследованиям, лучший источник о финансах.
TommyCeT
| #
Назван простой напиток, который спасает от повышения сахара в крови https://x.com/Fariz418740/status/1904006505147629966
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://fm70.ru/
Donaldlaf
| #
darknet market links https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark web market urls
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://forma-doma.ru/
StormZ5
| #
Простой язык о финансах: https://ipoteka-rassrochka.ru
DragonZ3
| #
Использование на практике https://7sevenauto.ru финансов.
Avrora casino
| #
Avrora casino
A Brief History of Elemental Analysis » Artemis Analytical
Toliknaf
| #
darkmarket https://github.com/abacuslink4jjku/abacuslink – darkmarket link
Markcen
| #
Как выбрать надёжного застройщика? Полезные рекомендации — https://formatlight.ru/
Blade1366
| #
Как сделать финансы? https://korel-surgut.ru
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://forpost-ural.ru/
FaridBaile
| #
Наконец-то нашел идеальную кухню! “Эталон Кухни” – выполнили в срок, качественно. Звоните: 8-843-258-86-66 Посмотреть на карте
Rabyitabe
| #
dark web market https://github.com/darkwebsitesyhshv/darkwebsites – tor drug market
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://forstnpf.ru/
nvblfurn
| #
http://spbsseu.ru/page/pages/mir_onlayn_igr_dlya_malchikov__razvlechenie_i_razvitie.html игра противоположности для дошкольников 6 7
KeithCat
| #
iphone specifications iphone prices
Code3173
| #
Эксперты рекомендуют эти ресурсы https://remont-skupka.ru по финансам.
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://fort-pro.ru/
DragonZ6
| #
https://sapfir42.ru Как устроен механизм финансов?
DonDonfaita
| #
dark web link https://github.com/nexusdarkrtv1u/nexusdark – darknet drug store
NightV7
| #
Как выбрать лучшее решение для финансов: https://finpartners44.ru
Markcen
| #
Новостройки или вторичка? Полезные рекомендации — https://freski23.ru/
Philliptroli
| #
Прочные и удобные зип пакет опт и розница, разные размеры, надежная застежка. Для хранения, фасовки и перевозки. Оформите заказ онлайн с быстрой доставкой!
BediktUnlon
| #
На сайте https://orliman.shop вы найдете огромное количество ортопедических товаров, созданных для вашего здоровья. В каталоге представлены коленные, голеностопные ортезы, а также корсеты. В большом количестве находятся комфортные приспособления для фиксации. Также имеются бандажи, стельки, специальная обувь. Все это представлено в огромном ассортименте и по доступным ценам. Перед вами новинки, а также те товары, которые пользуются огромным спросом. В магазине постоянно проходят увлекательные акции.
Warrennapix
| #
Discover Trip to Montenegro crystal clear sea, mountain landscapes, ancient cities and delicious cuisine. We organize a turnkey trip: flight, accommodation, excursions.
RichardMog
| #
В Баку найдена одна из трех пропавших без вести несовершеннолетних подруг https://x.com/Fariz418740/status/1904141438733979773
RobertSania
| #
Индивидуалки Екатеринбурга Индивидуалки Екб ,Индивидуалки Екатеринбурга ,Индивидуалки Екатеринбург ,Проститутки Екб ,Проститутки Екатеринбурга ,Эскорт Екатеринбург ,Дешевые проститутки Екатеринбурга ,Дешевые проститутки Екб ,Шлюхи Екатеринбург ,Путаны Екатеринбург
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://fsk16.ru/
Donaldlaf
| #
darknet markets onion https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet sites
Light_Master
| #
Список инструментов по финансам: https://to-click.ru
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://fuseitdecore.ru/
IronZ5
| #
Друзья, поделитесь опытом по финансам! https://avto-arenda38.ru
cisco
| #
The Cisco Nexus 9300 series is perfect for scalable, secure networks.
We’ve been working with similar technologies—check it out
at Visit US.
Markcen
| #
Как выбрать надёжного застройщика? Читайте проверенные советы https://gala-park.ru/
Toliknaf
| #
dark markets https://github.com/abacuslink4jjku/abacuslink – darknet marketplace
ThunderZ4
| #
Авторитетное https://artkuhna.ru мнение о финансах.
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://galastroy-sk.ru/
Code4228
| #
Всем привет! Подскажите, где посмотреть информацию о финансах? https://maks-granit.ru
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://gameliongroup.ru/
mtsekaterinburgsig
| #
мтс тв екатеринбург
https://teachersconsultancy.com/employer/157233/24inetrnet-domashnij-ekb-2
провайдер мтс
poyufpailk
| #
Beterex – компания, которая обладает приличным опытом в решении любых задач. Мы лучшую логистическую цепочку выстроим, и ваши расходы на перевозку грузов из Китая снизим. Прозрачно работаем и оперативнее конкурентов. Взятые на себя обязательства гарантируем. Ищете жд перевозки из Китая? Mybeterex.com – портал, где более подробная информация о компании Бэтэрэкс представлена, советуем ее посмотреть уже сейчас. Предоставляем только качественные услуги. Найдем нужных вам производителей и поставщиков в Китае. Любой запрос ваш будет на все 100% осуществлен.
Pingrutty
| #
darknet sites https://github.com/aresonioncq0a7/aresonion – darknet market list
Rabyitabe
| #
dark web sites https://github.com/darkwebsitesyhshv/darkwebsites – dark market onion
GeorgeCon
| #
check this link right here now surf wallet
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://gavrilov-sergey.ru/
EagleReaper
| #
Как думаете, https://finance-alliance.ru где быстрее всего изучить финансы?
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://gazuslugi.ru/
DonDonfaita
| #
bitcoin dark web https://github.com/nexusdarkrtv1u/nexusdark – darknet links
Light959
| #
Как думаете, где лучше всего понять финансы? https://nikiforov-finance.ru
Alpha_Walker
| #
Простой язык о финансах: https://avtolombardbryansk.ru.
Billymep
| #
https://artcet.ru/
Markcen
| #
Инвестиции в жильё: с чего начать? Читайте проверенные советы https://geogroupeng.ru/
nvblfurn
| #
http://peling.ru/wp-content/pages/mir_priklucheniy__ogon_i_voda___igra_s_ogonkom.html сапер играть онлайн бесплатно без регистрации
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://geotech48.ru/
Donaldlaf
| #
tor drug market https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark web market list
RedStriker
| #
Давайте обсудим о финансах: https://skupka-52.ru
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные статьи и советы — https://geotop62.ru/
1win_pnMl
| #
1вин официальный сайт вход 1вин официальный сайт вход .
OmegaSpark
| #
Ответы на частые вопросы о финансах: https://solnce38.ru
1win_gySt
| #
1win зайти http://www.1win813.ru .
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://geovodservice.ru/
Toliknaf
| #
darknet market links https://github.com/abacuslink4jjku/abacuslink – dark markets 2025
Pingrutty
| #
darknet markets https://github.com/aresonioncq0a7/aresonion – darknet drug market
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://germes-alania.ru/
1win_vrMl
| #
1win kg скачать 1win6011.ru .
Thunder_Protocol
| #
Плюсы и минусы разных подходов к финансам: https://zaim-pod-zalogi.ru
IronGuardian
| #
Основы https://mdk-finance.ru финансов для новичков.
1win_xuSt
| #
1win rossvya https://1win813.ru/ .
WilliamIRrutty
| #
dark market onion dark market list
Markcen
| #
Инвестиции в жильё: с чего начать? Полезные рекомендации — https://ggs45.ru/
mostbet_caMa
| #
mostbet kg отзывы http://www.mostbet794.ru .
Rabyitabe
| #
darknet markets onion https://github.com/darkwebsitesyhshv/darkwebsites – dark web drug marketplace
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные рекомендации — https://ginloft.ru/
FNDaviditabe
| #
darknet websites dark web markets
Kxyufaita
| #
dark web market links darknet marketplace
ThomasVep
| #
Спасибо за красивые розы! Девушка была в восторге.
букет цветов томск
Warden1961
| #
Эксклюзивное интервью с экспертом по финансам: https://n-fanera.ru
1win_nbMl
| #
1win партнёрка http://www.1win6011.ru .
mostbet_keMa
| #
скачат мостбет https://mostbet794.ru/ .
Ghost_Nomad
| #
Доступно объяснено https://kpkperspektiva.ru о финансах.
DonDonfaita
| #
darkmarket 2025 https://github.com/nexusdarkrtv1u/nexusdark – darknet markets url
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://gismt72.ru/
1win_xrSt
| #
1wi https://www.1win813.ru .
Volodyanaf
| #
darkmarket link darkmarket list
Markcen
| #
Новостройки или вторичка? Читайте проверенные советы https://gk-olympic.ru/
IronX4
| #
Подборка ресурсов по финансам: https://galatur-perm.ru
PabloTon
| #
Planning a vacation? Podgorica airport is a warm sea, picturesque beaches, cozy cities and affordable prices. A great option for a couple, family or solo traveler.
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://glaskek.ru/
MarkNOlaf
| #
darknet markets onion darknet websites
Donaldlaf
| #
darknet market lists https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – onion dark website
GhostV5
| #
Новые https://lombard-zaklad.ru подходы в финансах.
Michaelhum
| #
Holidays in Tivat are European comfort at an affordable price. Transparent sea, clean beaches, historical cities and warm climate all year round.
Pingrutty
| #
best darknet markets https://github.com/aresonioncq0a7/aresonion – darknet markets
Markcen
| #
Новостройки или вторичка? Полезные статьи и советы — https://glass161.ru/
mostbet_cbMa
| #
motsbet mostbet794.ru .
DragonZ3
| #
Детальный анализ финансов: https://jaklin63.ru
Toliknaf
| #
dark markets https://github.com/abacuslink4jjku/abacuslink – onion dark website
Markcen
| #
Инвестиции в жильё: с чего начать? Узнайте подробности https://glatt-nsk.ru/
WilliamIRrutty
| #
darknet drug market darknet markets url
GeorgeTom
| #
Source:
casinorushplay.org
Sazruun
| #
Мы изготавливаем дипломы любых профессий по приятным тарифам. Стоимость может зависеть от определенной специальности, года выпуска и университета. Стараемся поддерживать для покупателей адекватную политику тарифов. Важно, чтобы дипломы были доступны для большого количества граждан. купить диплом продавца
LightCode
| #
Подробное https://vash-lombard.ru руководство по финансам.
Markcen
| #
Как выбрать надёжного застройщика? Узнайте подробности https://glavdecor-ekb.ru/
Bernieimist
| #
перенаправляется сюда http://maps.google.mk/url?q=https://www.google.com.na/url?sa=t&rct=j&q=&esrc=s&source=web&cd=11&ved=0ahUKEwin16O_he3KAhVDPBoKHXUcA8IQFghFMAo&url=https://iskprogress.ru/blog/geotehnicheskij-monitoring/
1win_liSa
| #
1win ваучер http://pboarders.borda.ru/?1-11-0-00000929-000-0-0-1742818701 .
SteelX1
| #
Эксклюзивное интервью с https://buhcentr-vlg.ru экспертом по финансам.
FNDaviditabe
| #
darknet market dark market list
Kxyufaita
| #
dark web link dark websites
Markcen
| #
Хотите разбираться в рынке недвижимости? Узнайте подробности https://glavsnab-gbi.ru/
Rabyitabe
| #
darkmarket 2025 https://github.com/darkwebsitesyhshv/darkwebsites – dark markets
Rezaimotcor
| #
Современные МФО предлагают займ без отказа под ПТС автомобиля без изъятия транспорта. Это удобно, если авто необходимо для работы или повседневных дел. Деньги выдаются быстро, без проверки кредитной истории.
1win_ddSa
| #
1win скачать kg https://pboarders.borda.ru/?1-11-0-00000929-000-0-0-1742818701/ .
Markcen
| #
Хотите разбираться в рынке недвижимости? Читайте проверенные советы https://glavtorgmsk.ru/
Night_Fang
| #
Практические советы для работы с https://kraft-leasing.ru финансами.
Wolf458
| #
Как сэкономить на финансах: https://24ufa-service.ru
DonDonfaita
| #
darknet market lists https://github.com/nexusdarkrtv1u/nexusdark – darknet site
Markcen
| #
Хотите сдать квартиру в аренду? Узнайте подробности https://globus-balakovo.ru/
mostbet_qroa
| #
мастбет https://shorts.borda.ru/?1-18-0-00000397-000-0-0 .
Gilbertdic
| #
код вилочного погрузчика
MarkNOlaf
| #
darknet drugs dark market list
Pingrutty
| #
dark web market list https://github.com/aresonioncq0a7/aresonion – bitcoin dark web
Markcen
| #
Как выбрать надёжного застройщика? Полезные статьи и советы — https://glwin.ru/
Volodyanaf
| #
dark market list dark market onion
1win_rzSa
| #
1win. com 1win. com .
DarkV5
| #
https://astfin.ru Коллеги, поделитесь опытом по финансам!
Storm_Protocol
| #
Полезные сервисы для работы с https://leasing-box.ru финансами.
Donaldlaf
| #
darknet links https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darkmarket link
Markcen
| #
Хотите сдать квартиру в аренду? Полезные рекомендации — https://goncharoff-victory.ru/
mostbet_bdoa
| #
мостбет скачать казино мостбет скачать казино .
Gilbertdic
| #
экскаватор погрузчик jcb 4cx в россии
WilliamIRrutty
| #
dark market link darkmarket list
Markcen
| #
Хотите разбираться в рынке недвижимости? Полезные статьи и советы — https://gor-bur.ru/
CyberHunter
| #
Подборка ресурсов по финансам: https://sarros.ru
Toliknaf
| #
dark markets https://github.com/abacuslink4jjku/abacuslink – dark web sites
SilverX4
| #
Лучшие инструменты для работы https://gk-transstroy.ru с финансами.
Kxyufaita
| #
dark web drug marketplace dark market onion
hMustafaVep
| #
Пионы — это всегда стильно и элегантно!
пионы купить
Iariorusp
| #
Приобрести документ университета можно в нашем сервисе. advokatitrebinje.com
Jameswew
| #
Новруз может быть объявлен официальным праздником в Турции https://x.com/Fariz418740/status/1904268540363915402
WilliamSmamb
| #
профильные стальные трубы proftruba-moscow.ru с полимерным покрытием. защита от коррозии для использования в агрессивных средах.
mostbet_esoa
| #
мостбет скачать казино http://www.shorts.borda.ru/?1-18-0-00000397-000-0-0 .
Cazrpwe
| #
Добрый день!
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по выгодным тарифам. Стоимость может зависеть от определенной специальности, года выпуска и образовательного учреждения: п»їtutdiploms.com/
Rabyitabe
| #
dark market link https://github.com/darkwebsitesyhshv/darkwebsites – darknet markets 2025
Uazrbny
| #
Приветствую!
Для некоторых людей, заказать диплом университета – это необходимость, удачный шанс получить достойную работу. Однако для кого-то – это понятное желание не терять время на учебу в университете. С какой бы целью вам это не понадобилось, наша компания готова помочь. Максимально быстро, профессионально и по доступной цене изготовим документ любого года выпуска на настоящих бланках со всеми необходимыми печатями.
Ключевая причина, почему многие люди покупают документ, – желание занять хорошую работу. Предположим, знания дают возможность кандидату устроиться на привлекательную работу, а подтверждения квалификации нет. Если для работодателя важно наличие “корочек”, риск потерять вакантное место довольно высокий.
Приобрести документ о получении высшего образования вы имеете возможность в нашем сервисе. Мы предлагаем документы об окончании любых ВУЗов России. Вы сможете получить необходимый диплом по любой специальности, включая документы СССР. Даем 100% гарантию, что при проверке документа работодателем, никаких подозрений не возникнет.
Ситуаций, которые вынуждают приобрести диплом о среднем образовании достаточно. Кому-то очень срочно потребовалась работа, и нужно произвести впечатление на начальство на протяжении собеседования. Некоторые желают устроиться в большую компанию, чтобы повысить собственный статус в обществе и в дальнейшем начать свое дело. Чтобы не тратить массу времени, а сразу начать удачную карьеру, используя имеющиеся навыки, можно заказать диплом в интернете. Вы станете полезным для социума, получите денежную стабильность максимально быстро и просто- аттестат купить за 9 класс
Overlord5772
| #
Полезные сервисы для работы с финансами: https://nogataprofi.ru
JeffryZeque
| #
Портал для любознательных https://vseduxi.ru/sekretnyy-yazyk-podiuma-chto-daet-angliyskiy-fashion-guru/ Полезная информация, тренды, обзоры, советы и много вдохновляющего контента. Каждый день — повод зайти снова!
Sazrqan
| #
Приобрести диплом о высшем образовании !
Покупка диплома ВУЗа РФ в нашей компании – надежный процесс, ведь документ заносится в реестр. При этом печать выполняется на специальных бланках, установленных государством. Заказать диплом о высшем образовании arenadiplom24.online/vuzy/uralskij-finansovo-yuridicheskij-institut
Sazremn
| #
Приобрести диплом университета по невысокой стоимости возможно, обратившись к надежной специализированной фирме. Мы оказываем услуги по производству и продаже документов об окончании любых университетов России. Заказать диплом о высшем образовании– diploma-groups24.ru/kupit-diplom-v-gorode/gelendzhik.html
Night160
| #
Видеоуроки по финансам: https://f-m-a.ru
Walker1729
| #
Предлагаю подискутировать о финансах: https://aspire-capital.ru
Pingrutty
| #
onion dark website https://github.com/aresonioncq0a7/aresonion – dark markets
DonDonfaita
| #
darknet markets 2025 https://github.com/nexusdarkrtv1u/nexusdark – tor drug market
MarkNOlaf
| #
dark market url tor drug market
Williemem
| #
Добрый день!
Один из популярных вопросов среди пользователей — как выбрать лучший ноутбук для работы или игр? На информационных сайтах можно найти подробные обзоры самых актуальных моделей. Здесь рассказывается о характеристиках ноутбуков, таких как процессор, память, графика и другие важные параметры. Также рассматриваются аспекты, которые важно учитывать при выборе устройства, будь то для учебы, работы или игр. Эти сайты помогут вам выбрать оптимальный ноутбук в зависимости от ваших нужд.
Как преодолеть страх публичных выступлений? Начните с практики перед зеркалом или перед друзьями. Чем больше вы будете тренироваться, тем уверенно будете себя чувствовать на сцене.
Больше информации по ссылке – https://asimutaero.ru/
как сделать черный краситель, видео интересные истории, лучшие недорогие наушники беспроводные
интересные факты фиджи, лучшие ироничные детективные сериалы, интересные факты про вулканы
Удачи!
SilverStriker
| #
Как вам этот ресурс о финансах? https://yuvelir-versal.ru
Volodyanaf
| #
darkmarkets darknet drug market
Donaldlaf
| #
dark markets https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark web markets
DragonZ9
| #
Простой язык о финансах: https://1centr-prodazhi.ru
EagleX3
| #
Секреты https://success-finance41.ru профессионалов в финансах.
Toliknaf
| #
dark web marketplaces https://github.com/abacuslink4jjku/abacuslink – darknet market list
Petuvhnig
| #
На сайте https://kometagaming.site/ мы собрали рейтинг официальных букмекерских контор с лицензией, которые позволяют легально делать ставки на спорт и где будет гарантирована честная игра и своевременные выплаты. Мы решили этот вопрос и собрали лучшие компании в топ букмекерских контор. Лучшие официальные букмекерские конторы для ставок на спорт – это полезная информация для тех, кто хочет начать играть, а также получать различные бонусы и легкий вывод денег.
PhantomStriker
| #
Коллеги, поделитесь опытом https://lombardpysh.ru по финансам!
Robertemele
| #
Интернет-магазин дисков и шин Disks Tyres занимается розничной продажей шин и дисков на легковые автомобили грузовые авто и внедорожники https://disks-tyres.ru/
Pingrutty
| #
dark market onion https://github.com/aresonioncq0a7/aresonion – best darknet markets
rokogokag
| #
Ресурс torentino.org скачать игры через торрент вам предлагает. Предлагаем целый ряд конкурентных преимуществ. Наши игры постоянно обновляются. Комфортная система поиска дает возможность быстро найти то, что нужно. Насладитесь возможностями новейшей индустрии развлечений. Ищете скачать игру 2015? Torentino.org – здесь представлены разные игры, которые помогут скоротать время. Мы распределили их по множеству категорий, чтобы вам было удобно отыскать и скачать игру. Каждый файл обязательно проверяется на наличие вирусов. Оперативных вам загрузок!
Cyber48
| #
Предлагаю подискутировать о финансах: https://winfindmd.ru
RichardFub
| #
В «Фабрике остекления» мы предлагаем широкий выбор оконных конструкций для любых нужд. Наши пластиковые окна предназначены для остекления балконов, лоджий, квартир и загородных домов. Мы работаем только с высококачественными профильными системами, что позволяет создавать окна, которые идеально подходят для местных климатических условий. Наши окна обеспечивают отличную защиту от холода и шума, создавая комфортную атмосферу в вашем доме.
Master9344
| #
Шутки про https://spb-lombard.ru финансы.
Harryskync
| #
Добрый день!
Как создать здоровые привычки? Начните с маленьких шагов и делайте их постоянными. Используйте принцип 21 дня, чтобы новая привычка стала частью вашей жизни. Помните, что ключ к успеху — это последовательность и упорство.
Как стать более продуктивным на работе? Ставьте четкие цели, оптимизируйте рабочие процессы и избегайте отвлечений на посторонние вещи.
Больше информации по ссылке – https://asimuthaero.ru
как сделать разрешение свое, фейк паспорт как сделать, поздравления интересные
интересные бары москвы, как сделать викторину презентацию, как сделать сердце
Удачи!
Silver_Slayer
| #
Примеры успеха в финансах: https://ikmir.ru.
DonDonfaita
| #
dark web marketplaces https://github.com/nexusdarkrtv1u/nexusdark – dark markets 2025
Dragon827
| #
Какие платформы https://etcenrt.ru использовать для финансов?
Donaldlaf
| #
darknet drug market https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet websites
Matthewadare
| #
сервисы для покупки аккаунтов http://social-accounts-marketplace.ru
ArchieMog
| #
Добрый день!
Как повысить свою самооценку? Работайте над собой, ставьте реальные цели и добивайтесь их. Положительные аффирмации и благодарность за то, что вы уже достигли, также помогут улучшить самооценку.
Для начинающих растяжка — это отличная возможность улучшить гибкость и подготавливать тело к интенсивным тренировкам. Растяжка для начинающих поможет предотвратить травмы и улучшить подвижность. Упражнения на растяжку подходят для всех, кто хочет улучшить свое здоровье. Занимаясь растяжкой, вы можете заметно повысить уровень своей физической подготовки.
Больше информации по ссылке – https://ptello.ru/
интересные факты про шолохова, как сделать содовый раствор, как пишется поинтересней
задачи на логику интересные, как сделать лайфхак, рейтинг лучших вкладышей беспроводных наушников
Удачи!
Edwardadusy
| #
биржа для продажи аккаунтов маркетплейс готовых аккаунтов
Striker7014
| #
Лучшие инструменты для работы с финансами: https://eurekainvest.ru
Franktaf
| #
zooma casino зеркало
Blue420
| #
Всем привет! Подскажите, где https://limonykt.ru посмотреть информацию о финансах?
Toliknaf
| #
dark market 2025 https://github.com/abacuslink4jjku/abacuslink – dark web market list
RedGuardian
| #
Как устроен механизм финансов? https://head-bk.ru
Pingrutty
| #
darkmarket url https://github.com/aresonioncq0a7/aresonion – darknet market
Rabyitabe
| #
bitcoin dark web https://github.com/darkwebsitesyhshv/darkwebsites – dark web market links
Cyber_Walker
| #
Глубокий анализ https://sermusmax.ru финансов.
RedReaper
| #
Приветствую. Подскажите, где найти https://ic-gpp.ru информацию о финансах?
ojepaket
| #
Портал настроим-яндекс-директ.рф предоставляет квалифицированные услуги. Мы глубокими знаниями платформы Яндекс Директ располагаем. Предлагаем лучшую стратегию и учитываем особенности вашего бизнеса. Настроены на долгосрочные взаимоотношения. Ищете настройка и ведение яндекс директ? Xn—–6kcrbecsfrepkel5alfjkq8y.xn--p1ai – здесь представлены отзывы клиентов, ознакомиться с ними можете в любое время. Воспользуйтесь достоинствами ведения контекстной рекламы в Яндекс Директ с нашей командой. Поможем с максимальной эффективностью достичь ваших бизнес-целей!
Pioneer4514
| #
Лично мне помог этот https://cpk-garant.ru ресурс по финансам.
DonDonfaita
| #
darknet markets links https://github.com/nexusdarkrtv1u/nexusdark – dark market 2025
RichardFub
| #
«Фабрика остекления» предоставляет полный спектр услуг по остеклению квартир, коттеджей и коммерческих объектов. Мы используем только современные профильные системы и стеклопакеты, чтобы гарантировать тепло, уют и долговечность. Наши окна обеспечат отличную шумоизоляцию и защиту от внешних факторов.
StormWarden
| #
Какие платформы использовать для финансов? https://fs60.ru
kusegpor
| #
Компания «Отопление и водоснабжение» предоставляет профессиональные услуги по монтажу отопления. Мы предлагаем доступные цены. Сотрудничаем с ведущими изготовителями оборудования. Готовы создать теплый дом для вас и вашей семьи. Ищете монтаж водоснабжения? Santex-uslugi.ru – портал, где вы заявку можете оставить. Работаем с новейшим оборудованием и передовыми технологиями. Используем высококачественные материалы, чтобы гарантировать долговечность вашей системы отопления. Вы можете проконсультироваться с нами по интересующим вопросам.
Pingrutty
| #
darknet markets https://github.com/aresonioncq0a7/aresonion – darknet markets url
DragonV9
| #
Простой язык о финансах: https://ligaexpert.ru
Toliknaf
| #
darkmarket 2025 https://github.com/abacuslink4jjku/abacuslink – darkmarket url
Steel10
| #
Памятка https://larecgold.ru для работы с финансами.
Franktaf
| #
zooma casino официальный
Rabyitabe
| #
darkmarket list https://github.com/darkwebsitesyhshv/darkwebsites – darkmarket list
BlueWalker
| #
Преимущества разных https://naabinvest.ru подходов к финансам.
Vortex70
| #
Полное руководство по финансам от А до Я: https://ipoteka-sf.ru
DonDonfaita
| #
dark web market urls https://github.com/nexusdarkrtv1u/nexusdark – darkmarkets
Eagle_Slayer
| #
Лично мне помог https://rozalya.ru этот ресурс по финансам.
Samuellok
| #
Здравствуйте!
Купить сигареты Кент Блю 1000 (мрц200) — это выбор для тех, кто предпочитает мягкие и легкие сигареты с свежим вкусом. Сигареты Кент Блю 1000 подарят вам освежающее курение с легким и приятным ароматом. Заказывайте сигареты Кент Блю 1000 (мрц200) с доставкой на дом. Эти сигареты идеально подходят для любителей легких и свежих ароматов. Сделайте покупку и наслаждайтесь курением!
Купить сигареты Кент кристал синий – 950 (мрц169) — это выбор для тех, кто ищет более мягкие сигареты с необычным вкусом. Сигареты Кент кристал синий 950 подарят вам невероятно гладкое курение с легкими нотками свежести. Вы можете купить сигареты Кент кристал синий – 950 (мрц169) с быстрой доставкой в любой уголок города. Они подойдут для тех, кто хочет наслаждаться качественными сигаретами каждый день. Закажите Кент кристал синий прямо сейчас!
Лучшие сигареты по ссылке – https://t.me/sigaretikupit_ru/, канал в telegram – @sigaretikupit_ru
chapman сигареты classic купить, Купить сигареты Минск нано 850, мальборо сигареты зеленые купить
Сигареты Ротманс нано 950 (мрц185), купить сигарету электронную в украине, Купить сигареты Минск компакт син/красн – 900
Удачи за сигареткой!
BillyWes
| #
Добрый день!
Вопросы выбора и использования умных часов часто обсуждаются на информационных сайтах. Здесь можно найти обзоры популярных моделей, таких как Apple Watch, Samsung Galaxy Watch и других. Сайты подробно рассказывают, как выбрать умные часы в зависимости от их функций и совместимости с другими устройствами. Также рассматриваются вопросы о том, как использовать умные часы для мониторинга здоровья и фитнес-активности. Эти ресурсы помогут вам выбрать идеальные умные часы для вашего стиля жизни.
На информационных сайтах можно найти много полезной информации о различных гаджетах для путешественников. В статьях рассматриваются устройства, которые помогут вам в поездках, такие как портативные зарядные устройства, наушники с шумоподавлением и другие аксессуары. Сайты предлагают советы по выбору гаджетов, которые облегчат вашу жизнь в дороге. Также рассматриваются вопросы о том, как выбрать гаджеты для комфортного путешествия и работы в пути. Эти ресурсы помогут вам собрать идеальный набор гаджетов для путешествий.
Больше информации по ссылке – https://onello.ru/
как сделать вкусно макароны, фильмы комедии интересные, как сделать повышенное давление
самый страшный фильм психологический ужасов, почему телефон стал быстро разряжаться, как маникюр сделать дома
Удачи!
Gilbertdic
| #
обзор фронтального погрузчика
Pingrutty
| #
darknet websites https://github.com/aresonioncq0a7/aresonion – darkmarket
EaglePioneer
| #
Какие изменения в финансах? https://dominvestment.ru
Donaldlaf
| #
darknet markets https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet market lists
Robertemele
| #
Интернет-магазин дисков и шин Disks Tyres занимается розничной продажей шин и дисков на легковые автомобили грузовые авто и внедорожники https://disks-tyres.ru/
Jeffreymak
| #
Добрый день!
Как научиться планировать бюджет? Определите свои доходы и расходы, следите за расходами и ставьте цели на экономию, чтобы управлять своими финансами эффективно.
Как научиться быть терпеливым? Работайте над собой, примите, что не все можно получить сразу, и старайтесь сохранять спокойствие в сложных ситуациях.
Больше информации по ссылке – https://tyrtsia.ru
гендер пати как сделать, айти профессии самые высокооплачиваемые, интересные русские сериалы новые
как сделать кудри африканские, как сделать правильно тефтели, как сделать поджарку
Удачи!
DarkBlade
| #
https://arenda-lizing.ru Коллеги, поделитесь опытом по финансам!
GeorgeTom
| #
Source:
– casinorushplay.org
Toliknaf
| #
darkmarket url https://github.com/abacuslink4jjku/abacuslink – dark markets 2025
EagleV3
| #
Нюансы https://kpk-mbk.ru работы с финансами.
OmegaV6
| #
https://teknos59.ru Чек-лист для работы с финансами.
KurersSnums
| #
На сайте http://umk-trade.ru изучите новости автомира, ознакомьтесь с тест-драйвами автомобилей различных марок, чтобы принять правильное решение о покупке. Вы узнаете обо всех технических характеристиках, особенностях каждой модели. Есть тест-драйв автозапчастей Hordano, шин и дисков, которые крайне важны в эксплуатации автомобиля. Кроме того, есть информация по поводу того, стоит ли брать Мерседес в аренду, какие преимущества вы получите от этого. Любопытные, увлекательные статьи появляются регулярно.
Rabyitabe
| #
darknet sites https://github.com/darkwebsitesyhshv/darkwebsites – darknet markets 2025
FNDaviditabe
| #
darknet markets links dark market
Phantom30
| #
Полное руководство по финансам от А до Я: https://debetum-online.ru
Pingrutty
| #
dark market 2025 https://github.com/aresonioncq0a7/aresonion – darknet links
Phantom161
| #
Как считаете, где лучше всего изучить финансы? https://tandemkreditnn.ru
DonDonfaita
| #
darknet market https://github.com/nexusdarkrtv1u/nexusdark – darknet markets onion
AlphaX9
| #
https://buhusfin.ru Перспективы финансов.
Overlord4086
| #
Кто-нибудь ориентируется в финансах? https://finansist11.ru Куда зайти?
MarkNOlaf
| #
dark markets 2025 tor drug market
MartyRow
| #
Здравствуйте!
На информационных сайтах для любителей технологий можно найти подробные руководства по сборке и настройке ПК. В статьях пошагово объясняется, как выбрать компоненты для компьютера, а также как собрать его самостоятельно. Сайты предлагают советы по выбору процессора, видеокарты, материнской платы и других деталей. Также представлены рекомендации по оптимизации работы ПК для игр или профессиональной деятельности. Это полезная информация для тех, кто хочет создать персональный компьютер, подходящий под его нужды.
Если вы увлекаетесь фотографией, информационные сайты предлагают советы по выбору гаджетов для фотосъемки. В статьях рассматриваются камеры, объективы, дронов и другие устройства, которые помогут вам делать качественные фотографии. Сайты предлагают рекомендации по выбору подходящего оборудования для разных типов съемки, будь то пейзаж, портрет или макросъемка. Эти ресурсы помогут вам создать лучшие снимки с помощью новейших технологий.
Больше информации по ссылке – https://azimuhtaero.ru
как сделать самодельную бомбочку, как сделать атласную розу, как сделать объемную
интересные книги скачать бесплатно, как сделать колонку блютуз, как сделать авито про
Удачи!
Donaldlaf
| #
dark market url https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark market list
WilliamIRrutty
| #
darknet market list tor drug market
SilverX9
| #
Шутки про финансы: https://jet-ready.ru
MichaelMep
| #
Пластиковые окна от «Окна Мастер СПб» – это максимум комфорта и минимум хлопот. Мы используем лучшие профильные системы и исключаем любые недочёты на производстве.
Toliknaf
| #
darknet sites https://github.com/abacuslink4jjku/abacuslink – onion dark website
RobertDaw
| #
Non Gamstop casinos no deposit deals are a game-changer—wow!
non gamstop casinos 2020
Voyager4412
| #
Обязательно посмотрите этот материал о финансах: https://cf-mfo.ru
ShadowV1
| #
https://afk-zalog.ru Технические нюансы финансов.
Volodyanaf
| #
dark market list dark market
FNDaviditabe
| #
darknet markets links dark market list
Xafonunors
| #
На сайте https://strah.shop/ почитайте информацию на тему финансов, страховки и многого другого. Вы узнаете о том, как простимулировать семьи к тому, чтобы они повысили рождаемость и захотели родить второго, третьего ребенка. Есть советы по поводу того, как защитить себя от карманников. Есть публикации на тему того, как правильно и грамотно управлять своими долгами. Здесь постоянно появляются свежие записи, которые будут интересны всем. На сайте также имеются статьи на другие темы, интересные россиянам.
HintoBor
| #
Looking for the best cryptocurrency exchange rates? Discover the Coinxes cryptocurrency exchange https://coinxes.io/ – the best rates, the lowest commissions. The Coinxes platform is a digital exchange that allows users to buy and sell cryptocurrencies from anywhere in the world. Unlike a regular exchange, it boasts a large number of useful features.
Pingrutty
| #
darkmarket list https://github.com/aresonioncq0a7/aresonion – darknet drug store
1win_ovor
| #
1win официальный сайт вход http://boardwars.forum24.ru/?1-10-0-00000406-000-0-0 .
Rabyitabe
| #
dark websites https://github.com/darkwebsitesyhshv/darkwebsites – dark web drug marketplace
Silver677
| #
Давайте обсудим о финансах: https://lombard26.ru.
1win_baor
| #
1win войти https://boardwars.forum24.ru/?1-10-0-00000406-000-0-0 .
DonDonfaita
| #
darknet market https://github.com/nexusdarkrtv1u/nexusdark – bitcoin dark web
Hunter6748
| #
Последние обновления по https://zaympts42.ru финансам.
MarkNOlaf
| #
dark web drug marketplace darknet drug store
1win_tjsr
| #
1вин кыргызстан 1win6012.ru .
1win_vwSn
| #
1вин войти http://1win814.ru/ .
Omega_Warden
| #
https://bkrural.ru Забавные моменты в финансах.
1win_cfor
| #
сайт 1win официальный сайт вход http://www.boardwars.forum24.ru/?1-10-0-00000406-000-0-0 .
mostbet_ampr
| #
мостбет казино войти http://tagilshops.forum24.ru/?1-4-0-00000205-000-0-0/ .
DonnellAlock
| #
Крайне советую сантехник Краснодар
MichaelMep
| #
Компания «Окна Мастер» из Санкт-Петербурга работает по принципу: “качество прежде всего”. Мы не допускаем брака и гарантируем точную геометрию каждого окна.
Steel932
| #
Практическое https://triumfi.ru применение финансов.
1win_rysr
| #
1вин вход с компьютера https://1win6012.ru .
1win_qfSn
| #
1win букмекер http://www.1win814.ru .
Donaldlaf
| #
darknet markets url https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark market onion
WilliamIRrutty
| #
dark web market dark market
gejufdlycle
| #
Ищете магазин лепнины? Piterra.ru/catalog/lepnina/ можно для разного стиля интерьера. Современная лепнина органично вписывается в пространство, оживляя стены, потолки, скрывая провода и электрические розетки, а также придавая выразительности помещению с помощью различных оттенков краски.
mostbet_chpr
| #
most bet http://www.tagilshops.forum24.ru/?1-4-0-00000205-000-0-0 .
mostbet_fnol
| #
mostbet официальный сайт mostbet официальный сайт .
FNDaviditabe
| #
dark markets 2025 dark web marketplaces
Kxyufaita
| #
tor drug market dark markets
уборка после ремонта спб
| #
Безупречный клининг в Санкт-Петербурге: Ваш неизменный партнер по наведению порядка!
Наша компания более 10 лет оказывает услуги клининга в Санкт-Петербурге и зарекомендовала себя как надежный партнер. Мы гордимся опытом нашей команды, которая состоит из высококвалифицированных специалистов, прошедших обучение и имеющих все необходимые сертификаты. Для нас важна не только оперативность, но и наивысшее качество выполняемых услуг.
Не ждите, пока грязь и беспорядок станут катастрофой. Дайте нам шанс вернуть вашему пространству чистоту и порядок! Узнайте всё о наших услугах и оставьте заявку на сайте : https://uberu21.ru/
Toliknaf
| #
dark web market list https://github.com/abacuslink4jjku/abacuslink – dark market link
Nomad9184
| #
https://avtolombard-odin24.ru Доступно объяснено о финансах.
Pingrutty
| #
dark market https://github.com/aresonioncq0a7/aresonion – darknet markets onion address
mostbet_eyol
| #
мостбет скачать бесплатно mostbet795.ru/ .
BlueZ9
| #
Интерактивный курс по финансам: https://spbbulat.ru.
1win_lysr
| #
1 win pro http://1win6012.ru/ .
Michaelkep
| #
https://rowe-av.ru/
1win_ofSn
| #
1win ракета https://1win814.ru/ .
mostbet_pmpr
| #
мостбет вход http://www.tagilshops.forum24.ru/?1-4-0-00000205-000-0-0 .
Omega601
| #
https://alfa55.ru Креативное решение к финансам.
Rabyitabe
| #
dark market list https://github.com/darkwebsitesyhshv/darkwebsites – darkmarket 2025
OrlandoVor
| #
Доброго!
Какие шаги помогут повысить свою продуктивность? Начинайте день с составления плана, выделяя время на важные задачи. Разбивайте крупные задачи на более мелкие и не забывайте делать перерывы, чтобы избежать перегрузки. Технологии, такие как тайм-трекинг, также могут помочь вам оставаться организованным.
Для того чтобы сделать скриншот на айфоне, достаточно нажать две кнопки одновременно. Это простой способ запечатлеть информацию или кадр с экрана. Как на айфоне сделать скриншот — это вопрос, который многие задают. Но ничего сложного в этом нет, и вы сможете легко научиться делать снимки экрана.
Больше информации по ссылке – https://akhobeda.ru
интересный русский детектив, полис омс как сделать, крокодил игра слова интересные
российские сериалы список лучших детективные, как фото сделать прозрачным, овсяное молоко как сделать
Удачи!
hacobBar
| #
Ищете купить обои с доставкой? piterra.ru/catalog/oboi – широкий выбор обоев в СПб и МСК. В нашем каталоге вы отыщите обои на кухню, обои под покраску, обои на стену. Вы можете купить обои в интернет-магазине или выбрать в салонах по адресам. У нас на обои приемлемые цены, широкий выбор и удобная доставка. Не знаете, где найти магазин обоев в МСК либо СПб? Ознакомьтесь с нашим каталогом, сравните цены и подберите для вашего интерьера идеальные обои!
Vortex885
| #
Эксклюзивное интервью с https://exclusivebrn.ru экспертом по финансам.
mostbet_qmol
| #
мастбет mostbet795.ru/ .
Stewartiniva
| #
мобильная версия риобет зеркало riobet
DonDonfaita
| #
darkmarkets https://github.com/nexusdarkrtv1u/nexusdark – dark markets
Albertcoile
| #
продажа аккаунтов социальных сетей маркетплейсы для покупки аккаунта
казино с моментальными выводами
| #
казино с моментальными выводами
A Brief History of Elemental Analysis » Artemis Analytical
IronWarden
| #
Список инструментов по финансам: https://nacburo.ru
Jariorhje
| #
Где заказать диплом по нужной специальности?
Наша компания предлагает быстро и выгодно приобрести диплом, который выполнен на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями. Данный документ пройдет лубую проверку, даже с использованием специального оборудования. Решите свои задачи максимально быстро с нашим сервисом. Приобрести диплом ВУЗа! paning.flybb.ru/viewtopic.php?f=7&t=1853
Mazrwss
| #
Мы предлагаем дипломы любой профессии по доступным ценам. Стараемся поддерживать для заказчиков адекватную политику цен. Для нас важно, чтобы дипломы были доступными для большого количества граждан.
Приобретение диплома, подтверждающего обучение в университете, – это выгодное решение. Купить диплом любого ВУЗа: okdiplom.ru/kupit-diplom-novogo-obraztsa-9/
Sazrolx
| #
Студенческая жизнь прекрасна, пока не приходит время писать диплом, как это случилось со мной. Не стоит отчаиваться, ведь существуют компании, которые помогают с написанием и защитой диплома на высокие оценки!
Сначала я искал информацию по теме: купить дипломы в ставрополе недорого, купить оригинальность диплома, купить программное обеспечение для заполнения дипломов спо, куплю диплом высшего образования форум, куплю диплом о высшем образовании читать, а потом наткнулся на diplomybox.com/diplom-magistra
ShadowVoyager
| #
Доступно объяснено о финансах: https://dengiyest.ru
Alpha_Sentinel
| #
Мнение https://pravohelp23.ru специалистов о финансах.
Donaldlaf
| #
dark websites https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark web markets
Timothyner
| #
Zalogowalem sie na strone nayttely i od razu tego pozalowalem! Partie bez przerwy zacinaja sie, menu jest koszmarnie nieintuicyjny, a reklamy sa wszedzie. Mozna pomyslec, jak gdyby serwis byla zaniedbana – zero wsparcia, usterki na kazdej stronie, a dzialanie jest koszmarnie wolne. Informacji praktycznie brak, a sa one nieaktualne. Jesli chcesz bez problemow grac w brydza, wybierz alternatywne serwisy – tu nie znajdziesz nic wartosciowego!
Vortex_Slayer
| #
Повышение эффективности через финансы: https://yamal-stroy-invest.ru
Edwardwef
| #
Exponent Finance is redefining DeFi lending by providing secure, transparent, and high-yield investment solutions. Through smart contract-powered lending pools, Exponent Finance DeFi platform allows users to borrow and lend crypto assets with optimal efficiency and minimal risk. Whether you’re looking to earn passive income through staking or access instant liquidity, Exponent Finance offers a decentralized, non-custodial, and user-friendly solution to meet all your financial goals in the crypto ecosystem. https://exponent.ink
Dark_Walker
| #
Давайте обсудим о финансах: https://mikfinance.ru
Scottaxono
| #
Flaunch is the leading blockchain gaming launchpad, designed to help game developers and investors thrive in the Web3 gaming ecosystem. By offering secure token launches, NFT integrations, and decentralized crowdfunding, Flaunch enables game creators to fund, develop, and scale their projects with full transparency and community-driven support. Whether you’re a developer or an investor, Flaunch provides the tools to connect and grow in the blockchain gaming space. https://flaunch.tech
nvblfurn
| #
https://www.snabco.ru/fw/inc/geometry_dash__pogruzghenie_v_mir_geometricheskih_priklucheniy.html обвести рисунок по точкам для детей 7 8 лет
DonaldPauch
| #
Купить бонг
KennethGab
| #
Cytonic is revolutionizing blockchain security with advanced cybersecurity solutions tailored for Web3 applications. By integrating decentralized encryption, AI-powered threat detection, and smart contract auditing, Cytonic ensures maximum protection against cyber threats. Whether you’re securing DeFi protocols, NFTs, or enterprise blockchain systems, Cytonic’s cutting-edge security technology provides the highest level of data integrity and protection. https://cytonic.cc
Rabyitabe
| #
darknet drug store https://github.com/darkwebsitesyhshv/darkwebsites – dark markets 2025
Blue142
| #
Секреты профессионалов в финансах: https://biznes-curs.ru
DonDonfaita
| #
darknet markets onion address https://github.com/nexusdarkrtv1u/nexusdark – dark market url
DragonBlade
| #
Вебинар о финансах: https://stgold.ru.
AlbertSom
| #
Откровенно, сначала было ощущение, — ну, типичное казино. А потом незаметно.
Интерфейс удобный, всё реактивное, не нужно путаться в кнопках, чтобы запустить игру.
Темы очень разнообразные — от знакомых до тех, где глаза разбегаются.
Финансы заливал — зашли сразу, забирал — получил быстро, аж удивился, что всё чётко.
Техподдержка тоже не подводят: обратился, через короткое время уже всё решили.
В целом, казино Вулкан — неожиданно хорошая площадка. Не идеально, но честно, и местами даже весело. Главное — держать в голове, что всё должно быть в меру.
Pingrutty
| #
darknet market links https://github.com/aresonioncq0a7/aresonion – darkmarket link
GhostV9
| #
Малоизвестные фишки финансов: https://otdelresheniy.ru
ChrisWrard
| #
Elara Finance is transforming decentralized lending by offering secure, transparent, and flexible crypto loan solutions. Built on blockchain technology, Elara Finance enables users to borrow and lend digital assets seamlessly without intermediaries. With low-interest rates, automated smart contracts, and a permissionless DeFi environment, Elara Finance is making crypto lending accessible and profitable for investors worldwide. https://elara.ink
ZavuhgoW
| #
Топ букмекерских контор для ставок на сайте https://pkrdomgaming.site/ – это лучшие официальные букмекерские конторы для ставок на спорт. Гарантирована честная игра и своевременные выплаты. Мы решили этот вопрос и собрали лучшие компании в топ букмекерских контор. Лицензия, отличные коэффициенты, простота ввода и вывода денег.
Donaldlaf
| #
darknet market links https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark market url
Iron415
| #
https://logalk.ru Как сэкономить на финансах.
RafaelFer
| #
DEQ Finance is revolutionizing decentralized trading by offering a seamless, secure, and efficient crypto exchange experience. Built with cutting-edge blockchain technology, DEQ Finance provides traders with fast transaction speeds, deep liquidity, and a transparent trading environment. Whether you’re a beginner or a professional trader, DEQ Finance delivers high-performance DeFi solutions tailored to modern trading needs. https://deq.li
Dragon_Guardian
| #
Видел прогнозы, что Финансы будет главным трендом. Соглашусь? https://a-nalogi-a.ru
AllenTaulk
| #
Ремонт помещений https://msksfera.ru/remont-gostinicz/ под ключ: офисы, отели, склады. Полный комплекс строительно-отделочных работ, от черновой отделки до финишного дизайна.
Toliknaf
| #
dark web drug marketplace https://github.com/abacuslink4jjku/abacuslink – dark web market urls
Light838
| #
Эксперты рекомендуют эти ресурсы https://moslomb.ru по финансам.
Light_Overlord
| #
Эксклюзивное интервью с https://char21.ru экспертом по финансам.
Rabyitabe
| #
dark web drug marketplace https://github.com/darkwebsitesyhshv/darkwebsites – darknet markets onion
Iron_Spark
| #
Как устроен механизм финансов? https://pts59.ru
Pingrutty
| #
onion dark website https://github.com/aresonioncq0a7/aresonion – darkmarkets
DonDonfaita
| #
dark markets https://github.com/nexusdarkrtv1u/nexusdark – dark websites
ThomasVep
| #
Букет выше всех похвал! Очень доволен.
заказ цветов томск с доставкой
сайт Вован казино
| #
сайт Вован казино
A Brief History of Elemental Analysis » Artemis Analytical
burexTroky
| #
С t.me/mvavada вам скучать не придется. Официальный канал проекта Вавада предлагает атмосферу настоящего казино почувствовать. В VAVADA абсолютно каждый найдет игру по душе. Скорее к нам присоединяйтесь. Участвуйте в акциях и получайте шанс на дополнительные выигрыши. Испытайте удачу! https://t.me/mvavada – здесь всегда есть что-то интересное. Следите за обновлениями канала. В Вавада вас слоты с захватывающим геймплеем и неповторимым дизайном ждут. Играйте уже сегодня. Побеждайте вместе с VAVADA. Меняйте свою жизнь к лучшему!
RedX6
| #
Полное руководство по финансам от А до Я: https://kreditorium21.ru
SilverHacker
| #
Оптимизация https://light-gold.ru процессов через финансы.
Donaldlaf
| #
dark markets https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet links
ocenka-zagorod
| #
сбербанк оценка земельного участка http://ocenka-zagorod.ru
Storm94
| #
Полное руководство по финансам от А до Я: https://posuda-vo-dvore.ru
RobertDaw
| #
This non Gamstop casino has unreal graphics—totally blown away!
boku casino no gamstop
Toliknaf
| #
dark web market https://github.com/abacuslink4jjku/abacuslink – darknet marketplace
Kxyufaita
| #
darknet drug links tor drug market
WilliamIRrutty
| #
dark web sites dark web market urls
FNDaviditabe
| #
darknet sites dark markets 2025
Walker2835
| #
Советую качественный ресурс по https://proauto42.ru финансам.
GeorgeTom
| #
Source:
luckyspincasino.org
Master7006
| #
Как https://komisionka26.ru не сдаться при изучении финансов.
DavidAnync
| #
типография печать заказ https://tipografiya-poligrafiya2.ru
Aaronovexy
| #
типография печатной типография цены
Pingrutty
| #
dark web link https://github.com/aresonioncq0a7/aresonion – darknet sites
Volodyanaf
| #
darknet market darkmarkets
Rabyitabe
| #
darknet markets https://github.com/darkwebsitesyhshv/darkwebsites – onion dark website
fuel pressure sensor
| #
Great goods from you, man. I’ve understand your stuff previous to and you’re just extremely magnificent.
I really like what you have acquired here, certainly like what you’re stating
and the way in which you say it. You make it enjoyable and
you still care for to keep it sensible. I can’t wait to read far more from you.
This is actually a tremendous website.
Ghost_Walker
| #
Топовый ресурс по теме финансов: https://mat-kapital124.ru
DonDonfaita
| #
darknet drug links https://github.com/nexusdarkrtv1u/nexusdark – darkmarket list
GhostOverlord
| #
Подкаст о финансах: https://pvh-tyumen.ru
RobertDaw
| #
Non Gamstop casinos UK are my new go-to—super fun!
non uk casinos not on gamstop
Dark_Spark
| #
Ошибки новичков в финансах: https://taxandlex.ru
NewRetroCasino_bael
| #
New Retro Casino https://newretromirror.ru/ .
Kxyufaita
| #
dark web drug marketplace darknet markets links
WilliamIRrutty
| #
darkmarket url darknet market
Donaldlaf
| #
darknet markets url https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet drug links
LightZ6
| #
Эксклюзивное интервью с экспертом по финансам: https://lombardiago.ru
FNDaviditabe
| #
darknet site darknet sites
GilbeelUNLAT
| #
На сайте https://advelma.ru изучите огромное количество объявлений на самую разную тематику, включая автотранспорт, растения и животные, мобильные телефоны, мебель, интерьер и многое другое. Здесь также вы сможете и познакомиться с мужчиной либо женщиной. Всего на сайте представлено почти 150 рубрик. Если и вам нужно что-то продать, то выложите объявление на этом портале. Регулярно здесь добавляются новые, интересные предложения. Для того чтобы воспользоваться полным функционалом, нужно зарегистрироваться.
Toliknaf
| #
dark websites https://github.com/abacuslink4jjku/abacuslink – dark web link
Betfew
| #
من شروع کردم به بازی کردن کازینوی آنلاین Takbet و تا الان مشکل خاصی نداشتم.
ناوبری ساده و کاربرپسنده و بازیها سریع لود میشن.
من بیشتر از همه از بازیهای لایو کازینو خوشم اومد — آدرنالین رو بالا میبره.
همچنین یه جامعه پشتیبانی در تلگرام دارن اینجا: takbetplay](https://t.me/takbetplay) — برای دریافت بونوس و پشتیبانی خوبه.
چرخشهای رایگانش رو دوست داشتم.
کسی دیگه اینجا امتحانش کرده؟.
اگه راهنمایی یا تجربهای دارید، بگید.
Pingrutty
| #
darknet site https://github.com/aresonioncq0a7/aresonion – dark market onion
Voyager7188
| #
Ответы на частые вопросы о финансах: https://artcredit124.ru
Guardian2833
| #
https://nn7lombard.ru Преимущества разных подходов к финансам.
OmegaFang
| #
Продвинутые техники финансов: https://region-omsk.ru
Rabyitabe
| #
darknet market list https://github.com/darkwebsitesyhshv/darkwebsites – darknet markets 2025
Wubucelrip
| #
На сайте https://xn--80abeehcvkgdf2bbeq5an.xn--p1ai/ почитайте всю полезную и актуальную информацию о популярном онлайн-заведении Casino Kent. Вас ожидает сервис высокого уровня, огромное количество динамичных развлечений, щедрые бонусы, приятная атмосфера. И самое важное, что ресурс обязательно понравится как новичкам, которые только осваивают подобные заведения, так и профессионалам, которые находятся в поисках идеального места. Оно радует безупречным уровнем сервиса, можно задать вопросы, когда захотите.
Premium SEO links for sale
| #
Many thanks for the awesome facts enclosed within your blog,
what follows is a little test for your blog page viewers. Who said the following quote?
. . . .A lie gets halfway around the world before the truth has a chance to get its pants on.
DonDonfaita
| #
dark web link https://github.com/nexusdarkrtv1u/nexusdark – darkmarket url
ErnestoNow
| #
ауф казино
Warden8770
| #
Эксперты https://kgu-invest.ru рекомендуют эти материалы по финансам.
DragonSlayer
| #
Как применять финансы в бизнесе? https://finansist-msk.ru
WilliamIRrutty
| #
darknet links darknet drug links
Kxyufaita
| #
darkmarkets best darknet markets
casino Aurora
| #
Aurora Casino — это ваш идеальный выбор для захватывающих и выгодных
игр. Здесь вы найдете самые популярные игры,
включая слоты, настольные
игры и живое казино с реальными крупье.
Не забывайте про бонусы и регулярные акции,
которые сделают вашу игру еще более увлекательной.
Почему казино с бесплатными
играми стоит выбрать? Мы предоставляем высококачественные игры, защищенные
платёжные системы и гарантируем быстрые выплаты.
Наши игроки всегда могут быть уверены в честности игры и прозрачности всех
условий.
Когда лучше начинать в Aurora Casino?
Зарегистрируйтесь, чтобы уже сегодня
получить доступ ко всем возможностям и
бонусам. Вот, что вас ждет:
Вам будут предложены большие бонусы, как
только вы зарегистрируетесь.
Ежедневные акции и турниры.
Большой выбор слотов и настольных игр.
В Aurora Casino ваши мечты о больших выигрышах становятся
реальностью. https://aurora-diamondcasino.quest/
DennisgaP
| #
дешево заказать грузчиков грузчики заказать
KevinGaito
| #
голд казино
FNDaviditabe
| #
darknet markets 2025 darknet markets onion
MarkNOlaf
| #
darknet markets onion address darknet drug market
TerrySouse
| #
грузчики заказать https://toguchin.standart-express.ru
AlbertDiubs
| #
нужны грузчики для переезда нанять грузчиков
Jerrysiz
| #
Elevate your car’s functionality and appearance with our remarkable tuning options. Find the genuine ability of your car through bespoke advancements. Our professionals of experts provides incomparable quality and ingenuity. Check out our webpage https://precisionautobodyfrederick.com/ to understand extra, and start your venture towards motoring superiority today.
Volodyanaf
| #
darknet marketplace darkmarket link
Donaldlaf
| #
dark markets 2025 https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark market onion
Pingrutty
| #
dark web market list https://github.com/aresonioncq0a7/aresonion – darknet drug store
RobertDaw
| #
Best non Gamstop casino I’ve played at is a gem—cool!
are non gamstop casinos safe
PhantomZ9
| #
Эволюция развития финансов: https://mikrozaimdv.ru
Toliknaf
| #
dark web drug marketplace https://github.com/abacuslink4jjku/abacuslink – darkmarket 2025
AlphaProtocol
| #
История внедрения https://cbu63.ru финансов в компании.
Robertfus
| #
Доброго!
Как снизить уровень стресса? Для этого полезны расслабляющие практики, такие как медитация, йога или простое дыхательное упражнение. Осознанность и регулярный отдых помогут вам восстановить баланс и снизить стресс.
Как научиться прощать? Прощение — это процесс, который начинается с вас. Принятие того, что не все можно изменить, и отпускание обид помогает освободить сердце и душу. Прощение — это не только для других, но и для вашего внутреннего спокойствия.
Больше информации по ссылке – https://piano-quartet.ru
как сделать бесконечный двигатель, как сделать физраствор, как сделать карту озон
какие недорогие беспроводные наушники хорошие, турецкие сериалы самые интересные, интересные факты про крым
Удачи!
Marioskach
| #
Glory Casino app
1win_zmoi
| #
1win скачать последнюю версию 1win скачать последнюю версию .
1win_husr
| #
1win kg скачать https://www.1win6014.ru .
mostbet_lzKr
| #
казино онлайн kg kharkovbynight.forum24.ru/?1-15-0-00003047-000-0-0-1742814422 .
1win_gskl
| #
1win официальный сайт скачать http://yamama.forum24.ru/?1-11-0-00000459-000-0-0-1742818616 .
JesusIceme
| #
Привет всем!
Как развить эмоциональный интеллект? Слушайте свои эмоции и учитесь их распознавать. Работайте над эмпатией и стараясь понять чувства других. Управляйте своими реакциями в различных ситуациях.
Для начинающих растяжка — это отличная возможность улучшить гибкость и подготавливать тело к интенсивным тренировкам. Растяжка для начинающих поможет предотвратить травмы и улучшить подвижность. Упражнения на растяжку подходят для всех, кто хочет улучшить свое здоровье. Занимаясь растяжкой, вы можете заметно повысить уровень своей физической подготовки.
Больше информации по ссылке – https://alcogolizmstop.ru
как сделать основным гугл, интересные факты о атмосфере, ужасы смотреть интересные
смотреть русские интересные детективы, как сделать свою клавиатуру, интересные факты дания
Удачи!
Hacker2948
| #
Давайте обсудим о финансах: https://kombodrayv.ru
Rabyitabe
| #
darkmarket https://github.com/darkwebsitesyhshv/darkwebsites – darkmarket list
1win_wmoi
| #
1win.kg 1win.kg .
mostbet_geei
| #
aviator mostbet aviator mostbet .
1win_lzsr
| #
1win официальный сайт регистрация https://1win6014.ru/ .
mostbet_ooKr
| #
скачат мостбет скачат мостбет .
1win_lmkl
| #
1win.kg http://www.yamama.forum24.ru/?1-11-0-00000459-000-0-0-1742818616 .
WilliamIRrutty
| #
darknet sites darknet markets
DonDonfaita
| #
dark web sites https://github.com/nexusdarkrtv1u/nexusdark – darkmarket list
FNDaviditabe
| #
darknet markets links darknet market lists
WarrenWak
| #
https://tur-kazan.ru/
mostbet_edei
| #
mostbets https://www.mostbet6001.ru .
Pingrutty
| #
dark web markets https://github.com/aresonioncq0a7/aresonion – dark market list
Overlord4154
| #
Полное руководство по финансам от А до Я: https://micro-car.ru
Blue_Fang
| #
Какие платформы использовать для финансов? https://billum-zaim.ru
MatthewPeeda
| #
Здравствуйте!
Как научиться работать в команде? Будьте открытыми к сотрудничеству, уважайте мнения других и поддерживайте коллег в совместной работе.
На информационных сайтах можно найти массу полезных рекомендаций по выбору гаджетов для интимной жизни. В статьях рассматриваются устройства для повышения либидо, улучшения эрекции и снижения уровня стресса. Сайты предлагают советы по выбору гаджетов, которые помогут улучшить качество сна и настроить гормональный фон. Эти ресурсы помогут вам наладить отношения с партнером и повысить сексуальную активность.
Больше информации по ссылке – https://art-novosibirsk.ru/
как сделать ко, как сделать глазурь белую, самые лучшие зарубежные детективные сериалы
как дома сделать караоке, мужские интересные имена, сериалы детективные hbo лучшие
Удачи!
1win_dsoi
| #
ванвин https://www.1win815.ru .
1win_rdsr
| #
1вин сайт официальный 1вин сайт официальный .
mostbet_yeKr
| #
мостбет авиатор https://kharkovbynight.forum24.ru/?1-15-0-00003047-000-0-0-1742814422 .
1win_ofkl
| #
1win вход на сайт http://yamama.forum24.ru/?1-11-0-00000459-000-0-0-1742818616/ .
Donaldlaf
| #
darknet markets 2025 https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet markets
mostbet_exei
| #
мостбет войти https://www.mostbet6001.ru .
Toliknaf
| #
dark web markets https://github.com/abacuslink4jjku/abacuslink – darknet drug market
SilverSentinel
| #
Эксперты рекомендуют эти ресурсы по финансам: https://zolotopenza.ru
gates of olympus bonus buy
| #
excellent issues altogether, you just won a new reader.
What could you recommend in regards to your put
up that you simply made some days in the past?
Any positive?
Reaper2046
| #
Конкретные шаги для работы с финансами: https://spasskomi.ru
zaimpodzalogptsekbsig
| #
займ под залог автоломбард
avtolombard-11.ru/ekb.html
взять займ под залог авто
RobertDaw
| #
Best non Gamstop casino for slots is my new favorite—sweet!
non gamstop paypal casino
Volodyanaf
| #
darknet market list dark web sites
WilliamIRrutty
| #
darknet drug market darknet markets links
Kxyufaita
| #
dark market link dark markets 2025
Rabyitabe
| #
darkmarket url https://github.com/darkwebsitesyhshv/darkwebsites – dark market onion
FNDaviditabe
| #
dark markets darknet markets url
MarkNOlaf
| #
darknet markets onion dark markets
SilverV4
| #
Оптимизация процессов через финансы: https://realtor27.ru
Geraldalory
| #
Добрый день!
Как научиться ценить свое время? Устанавливайте четкие приоритеты, избегайте прокрастинации и уделяйте время только тем вещам, которые действительно важны для вас.
Как справиться с трудными ситуациями? Применяйте гибкость, ищите решения, не зацикливайтесь на проблемах и помните, что трудности — это часть пути к успеху.
Больше информации по ссылке – https://relation1.ru/
корсет как сделать, греческий йогурт как сделать, игры в стиме интересные
как из майнкрафта сделать, как можно сделать валентинку, как сделать букву а
Удачи!
Foleyelova
| #
Ищете надежных мастеров и бригаду без посредников? Посетите сайт https://poisk-pro.ru/ – это бесплатный подбор надежных исполнителей в сфере ремонта, строительства и других видов услуг от частных лиц и организаций. Более 30000 исполнителей в сфере строительства и ремонта, дизайна, бытовых услуг, ремонта техники и прочих услуг. Подробнее на сайте.
Pingrutty
| #
darknet sites https://github.com/aresonioncq0a7/aresonion – darknet market lists
DonDonfaita
| #
dark web market https://github.com/nexusdarkrtv1u/nexusdark – dark web drug marketplace
WarrenWak
| #
казань йошкар ола автобус экскурсия
Cyber42
| #
Новые подходы в финансах: https://86148.ru
Warden4981
| #
Интерактивный курс по https://metrikacredit.ru финансам.
Donaldlaf
| #
dark market 2025 https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darkmarket url
Robertpug
| #
Добрый день!
Как развить силу воли? Работайте над самодисциплиной, ставьте перед собой четкие цели и преодолевайте трудности на пути к их достижению.
Как быть более внимательным к себе? Развивайте осознанность, слушайте свои потребности и чувства. Регулярно уделяйте время себе: отдыхайте, медитируйте и заботьтесь о своем теле и разуме.
Больше информации по ссылке – https://psihfak.ru/
интересные хобби, как сделать сыр косичку, хорошие наушники маленькие беспроводные
растяжка стретчинг для начинающих, как сделать драг клик, шукшин факты интересные
Удачи!
Toliknaf
| #
darknet sites https://github.com/abacuslink4jjku/abacuslink – dark web sites
WilliamIRrutty
| #
tor drug market darkmarket link
Kxyufaita
| #
darknet marketplace dark web sites
Red122
| #
Видел прогнозы, что Финансы будет главным трендом. Соглашусь? https://topazrussia.ru
GhostX6
| #
Что https://zaim-pensioner.ru нового в финансах?
LuzehCon
| #
На сайте https://xn—-7sbbstdmdlcdhy.xn--p1ai/ вы сможете ознакомиться со всей необходимой информацией, касающейся майнинга и криптовалюты. Этот ресурс является свободным информационным и предлагает ознакомиться со всеми полезными рекомендациями. Только на этом сайте представлены самые точные и любопытные сведения, касающиеся цифровой валюты, криптовалюты и всего, что с ней связано. Представленная информация будет полезна и новеньким. Вас обязательно заинтересует и крипто библиотека, в которой есть много всего полезного.
FNDaviditabe
| #
darkmarket 2025 darknet links
Pingrutty
| #
dark web market urls https://github.com/aresonioncq0a7/aresonion – darknet markets url
https://jetton-casinoempire.lol/
| #
Welcome to Jetton Casino – a world of entertainment and big winnings.
We offer the best slot machines, table games, and exclusive bonuses
for all users. Join us and start your journey into the world of gambling right now.
What makes Jetton login special? Our players not only get access to top-tier games but
also enjoy favorable winning conditions. Here, you’ll find regular tournaments,
cashback, free spins, and personalized bonus offers.
A huge selection of slot machines, roulette, poker, and live
games.
Regular promotions and personalized offers.
Fast withdrawals and secure transactions without delays.
Competitions for the most passionate players with valuable prizes.
Discover the best gaming opportunities with Jetton Casino. https://jetton-casinoempire.lol/
Rabyitabe
| #
darkmarket https://github.com/darkwebsitesyhshv/darkwebsites – darknet drugs
RobertDaw
| #
Non-Gamstop casinos offer the best escape—highly recommend!
casino free spins no deposit not on gamstop
Marioskach
| #
Glory Casino Spribe
GregoryClile
| #
Единственный знак зодиака, который является магнитом для несчастий и проблем https://x.com/Fariz418740/status/1904737515858174078
Fang1876
| #
Подробный разбор финансов: https://skupka-noutbukov-orenburg.ru
DonDonfaita
| #
darknet market https://github.com/nexusdarkrtv1u/nexusdark – dark web sites
GeorgeTom
| #
Source:
luckyspincasino.org
Volodyanaf
| #
darknet markets onion address darkmarket
Thunder_Code
| #
Будущее финансов: https://avtodengy-pts.ru
Vocapves
| #
На сайте https://exkavatornews.ru/ почитайте содержательные и полезные материалы на тему лесозаготовительной техники. Здесь вы найдете ответ на вопрос, стоит ли приобретать китайскую технику, какое оборудование считается максимально эффективным. Есть информация и о дорожно-строительной технике и о том, как ей лучше пользоваться. Ознакомьтесь с инновационными технологиями в сфере дорожного строительства. Изучите мнения экспертов на данную тему. Регулярно здесь появляются новые, любопытные материалы, которые будут полезны.
Sentinel1907
| #
https://pal58.ru Памятка для работы с финансами.
Donaldlaf
| #
darkmarket url https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darkmarket link
WilliamIRrutty
| #
darknet markets links darknet drugs
FNDaviditabe
| #
dark web drug marketplace darknet drug store
Toliknaf
| #
darkmarket 2025 https://github.com/abacuslink4jjku/abacuslink – darknet markets links
Pingrutty
| #
dark market 2025 https://github.com/aresonioncq0a7/aresonion – dark web market urls
RebeccaTaund
| #
The Aviator game on Betika (https://betika-aviator.readme.io/reference/betika-aviator-a-thrilling-ride-to-fortune) is a high-stakes arcade-style game where instinct and speed decide your win.
The rules are easy to follow: a plane rises higher and higher, and as it flies, the multiplier increases.
Hit the stop button before the flight ends
Be greedy — and the bet vanishes.
That’s why this game is about strategy too.
With Betika, you get:
– a user-friendly interface
– fast withdrawals
– 24/7 support
– play on any device
Play anytime, anywhere — at home, at work, or on the go.
Sign up on Betika and make your first takeoff.
Betika Aviator – your flight to big wins!
NightV2
| #
Ответы на частые вопросы https://cashufa102.ru о финансах.
VortexX4
| #
Как выбрать https://mnr-1.ru лучшее решение для финансов.
Rabyitabe
| #
darknet market lists https://github.com/darkwebsitesyhshv/darkwebsites – tor drug market
RobertDaw
| #
Casino non Gamstop sites have the best promos—super fun!
non gamstop casinos uk free spins
DonDonfaita
| #
dark market link https://github.com/nexusdarkrtv1u/nexusdark – dark web sites
Cyber_Master
| #
Технические нюансы финансов: https://radiolomdv.ru
Steel253
| #
https://expert42.ru Как сэкономить на финансах.
Kxyufaita
| #
darkmarket url dark web link
WilliamIRrutty
| #
dark web market urls dark web market urls
jecifuger
| #
Ikostum предлагает купить мужской костюм недорого. В нашем интернет магазине регулярно распродажи и акции проводятся. У нас найдется костюм на любой вкус. Поможем вам с фасоном, тканью и цветом определиться, предложим чехол и галстук подходящий, а также проконсультируем о последних тенденциях. https://ikostum.com/ – тут можете посмотреть условия доставки в любое время. Мы сотрудничаем с ведущими белорусскими и российскими производителями мужской одежды. Регулярно ассортимент обновляем. Отменное качество продукции гарантируем.
MarkNOlaf
| #
darknet market dark web link
Pingrutty
| #
darknet drugs https://github.com/aresonioncq0a7/aresonion – darkmarket url
Donaldlaf
| #
darknet marketplace https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark market list
IronSlayer
| #
Базовые принципы финансов для https://raiseauto.ru новичков.
Toliknaf
| #
tor drug market https://github.com/abacuslink4jjku/abacuslink – darknet markets
Volodyanaf
| #
darknet websites dark markets 2025
Davidslate
| #
спарк ру новости Мир азартных игр в интернете непрерывно эволюционирует, и портал Спарк.ру остается надежным источником актуальных новостей и аналитики в этой сфере. Казино онлайн переживают период бурного роста, привлекая все больше пользователей благодаря удобству, разнообразию игр и привлекательным бонусам.
xutuyDaw
| #
Чат-бот на базе ИИ ChatGPT – профессиональный помощник, который может понимать запросы, поддерживать диалог и предоставлять индивидуальные решения. Цепляющий контент создавайте, продажи оптимизируйте и в 10 раз оперативнее задачи решайте. Сосредоточьтесь на самом главном. Ищете бесплатный чат gpt? Ai-gptbot.ru – сайт, где представлены отзывы, ознакомьтесь с ними. Тут рассказываем, кому чат GPT нужен. Все работает быстро и удобно. Цены удивляют приятно своей гибкостью и демократичностью. Не упускайте шанс попробовать AI-GPTbot прямо сегодня!
rexejChask
| #
Магазин «Adept Apple» продает оригинальные качественные товары с гарантией. Мы большой ассортимент аксессуаров Apple и гаджетов предлагаем. Обладаем значительным опытом и стали для тысячи клиентов надежным партнером. Стремимся ваше обслуживание сделать максимально приятным и удобным. Ищете айфон в Туле? Adeptapple.ru – здесь можете найти что-то для себя. Делаем доступной для каждого технику Apple. С удовольствием поможем подобрать устройство или аксессуар, которые идеально подойдут, именно вам. Для нас крайне важно доставить от покупки клиентам удовольствие!
NightVoyager
| #
Подборка ресурсов по финансам: https://primadonna-ekb.ru.
KevinWoutt
| #
вентиляция в частном доме
Rabyitabe
| #
darkmarket list https://github.com/darkwebsitesyhshv/darkwebsites – darknet sites
DragonVoyager
| #
Что нового в финансах? https://gu72.ru
Richardodoge
| #
маркетплейс готовых аккаунтов онлайн магазин аккаунтов
CarmenPooky
| #
Доброго!
Скриншот на айфоне можно сделать за считанные секунды, просто нажав две кнопки одновременно. Это поможет вам сохранить важную информацию или момент, который вам нравится. Для того чтобы сделать скриншот на айфоне, одновременно нажмите кнопку включения и кнопку увеличения громкости. После этого вы увидите изображение экрана в галерее.
Как научиться управлять своим временем? Определите, что для вас действительно важно, и сфокусируйтесь на этих задачах. Разделите свой день на блоки времени, чтобы избежать отвлечений. Не забывайте выделять время для отдыха и восстановления.
Больше информации по ссылке – https://ffactor.ru/
интересный разбор слова, как сделать красивый шрифт, растяжка воронеж для начинающих
как сделать лаваш мягким, какой нибудь интересный факт, мелодрамы новые интересные
Удачи!
SteelProtocol
| #
Детальный анализ финансов: https://taxoparkinmsk.ru
Vortex37
| #
Обязательно посмотрите этот https://komissionkatula.ru источник о финансах.
Kxyufaita
| #
dark market url onion dark website
WilliamIRrutty
| #
darknet market links dark web drug marketplace
DonDonfaita
| #
dark market url https://github.com/nexusdarkrtv1u/nexusdark – darknet markets onion address
IronV9
| #
Креативное решение к https://podzalog34.ru финансам.
Pingrutty
| #
darknet markets 2025 https://github.com/aresonioncq0a7/aresonion – darknet markets links
FNDaviditabe
| #
dark markets dark websites
MarkNOlaf
| #
onion dark website darkmarket url
camp-centr
| #
Продажа путёвок https://camp-centr.com/camps/type/yazykovoy.html. Спортивные, творческие и тематические смены. Весёлый и безопасный отдых под присмотром педагогов и аниматоров. Бронируйте онлайн!
SilverX6
| #
Креативное решение к финансам: https://skupka-zalog-obmen.ru
Reaper7454
| #
Эксклюзивное интервью с экспертом по финансам: https://zelta-invest.ru
Michaelgaile
| #
печать визиток стоит двухсторонняя печать визиток
AlphaX2
| #
Примеры успеха в финансах: https://pba55.ru
Overlord7102
| #
Предлагаю подискутировать о финансах: https://nadezhnii-kredit.ru
BlueX1
| #
Авторитетное мнение о финансах: https://standart-audit.ru
Donaldlaf
| #
dark market https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark market 2025
tokorunorp
| #
Бэтэрэкс – компания, предоставляющая консалтинговые, финансовые, а также юридические услуги. Поможем вам снизить затраты с Китаем на ведение бизнеса. Принимаем заказы на доставку, как от частных лиц, так и от компаний. Сопроводим в деловых поездках и на переговорах. https://mybeterex.com – тут можете форму заполнить, и мы в ближайшее время с вами свяжемся. Найдем и предложим на товар в Китае лучшую оптовую цену. С удовольствием открыть производство вам поможем. Договоримся о выпуске продукции и проведем контроль качества. Обращайтесь!
Andrewcaf
| #
Комплексное проектирование Услуги по аренде техники и строительству с логотипом компании – На протяжении многих лет компания “ТрансАвто№7” предоставляет в аренду дорожно-строительную технику, а также выполняет широкий спектр строительных и земляных работ. Мы работаем как с крупными корпорациями, так и с малым и средним бизнесом, обеспечивая высокое качество услуг по строительству дорог, благоустройству территорий и прокладке инженерных сетей.
Hunter3219
| #
Может кто знает ориентируется в https://goldmaster1.ru финансах? Куда зайти?
zaimpodptskazancoN
| #
автоломбард птс
avtolombard-11.ru/kazan.html
займ под залог автомобиля
StormNomad
| #
Как https://rassbery.ru преуспеть в финансах?
JeremyPycle
| #
https://vodkacoin.online/
Spark9061
| #
Может кто знает ориентируется в финансах? https://sarlombard.ru Куда зайти?
MicheleMouck
| #
Добрый день!
Как быть уверенным в себе? Развивайте самоуважение, принимайте себя с вашими сильными и слабыми сторонами. Каждый шаг к уверенности — это шаг к самосознанию и осознанию своей ценности.
Как научиться прощать себя? Признание своих ошибок и понимание, что все люди совершают их, помогает избавиться от чувства вины. Прощение себя — это путь к внутреннему покою.
Больше информации по ссылке – https://lala-express.ru
интересные мультики про машинки, короткие и интересные аниме, как сделать костер дейз
лучшие советские детективные сериалы, как сделать филадельфия роллы, фантастика самые интересные фильмы
Удачи!
1win_bkst
| #
скачать 1win с официального сайта 1win816.ru .
//zdjciaporno.com/
| #
Co To Jest Seks Oralny? Jaka laughter różnica
E ‘ en Seks Oralny, Fellatio, i drobniejsze Tums? Seks oralny wymaga partnera i niezależnie dd
tego, która pozycja działa najbardziej brutalnie.
Jeden z partnerów używa ust, warg lub języka, aby uformować penisa, pochwę lub orchid
arenosus partnera. Wiele sync używa move jako
sposobu na rozgrzanie się lub zjedzenie przekąski podczas stosunku, brew stymulacja ustna może zawsze odgrywać rolę w trakcie lub op, też.
Jak Działa Seks Oralny? Seks oralny daje Tobie i Twojemu partnerowi zwinny sposób na wklęsłodruk, z wyjątkiem regularnych harmonicznych san marinese.
Seks oralny joke tylko wtedy, gdy część phrase perform from nothing fishing registration. Jak
Działa Seks Oralny? Powszechnym aktem seksualnym wród sync w kadym wieku i kadej pci powszechnym aktem seksualnym
wród. Określany również jako giovanni boccaccio lub fellatio,
obejmuje ustną linię sukcesji upakowanych
komórek lub odbytu partnera. Może być dapat sama dotykowy,
jak samodzielny akt. https://git.uucloud.top/corinne49w1398
1win_bvmi
| #
1win кыргызстан http://1win6015.ru/ .
mostbet_kmKi
| #
мост бет http://maksipolinovtsu.forum24.ru/?1-1-0-00000194-000-0-0-1742815870/ .
Rabyitabe
| #
dark web sites https://github.com/darkwebsitesyhshv/darkwebsites – best darknet markets
Wolf_Blade
| #
Базовые принципы финансов для новичков: https://idance-school.ru
AlfredoFeend
| #
Odwiedzilem na witryne muodossa i przezylem szok. Szata graficzna sugeruje, jakby pochodzil sprzed dekady, dziala to dramatycznie, a merytorycznych danych prawie nie ma. Niektore sekcje sa puste, linki prowadza donikad, a wsparcie nie odpowiada. Falszywe informacje na stronie, serwis jest kompletnie bezuzyteczny! Jesli potrzebujesz sprawdzonych tresci o biegach, nie trac czasu na ten portal – ten serwis nic ci nie da.
1win_vjEn
| #
1 vin 1 vin .
Wolf_Master
| #
Изучите этот источник о финансах: https://cicred.ru
Kxyufaita
| #
darkmarket link dark market url
Iron578
| #
Факты и вымысел о финансах: https://armavir-avtolombard.ru
MatthewBoods
| #
Noon Capital is revolutionizing real estate crowdfunding by offering secure, decentralized, and high-yield investment opportunities. Through blockchain-powered property financing, Noon Capital investment platform allows investors to participate in fractional real estate ownership, earn passive income, and diversify their portfolios with full transparency and security. Whether you’re an institutional investor or an individual seeking real estate-backed DeFi solutions, Noon Capital provides a seamless and profitable investment experience. https://noon.ad
FrancisSah
| #
Kyros Finance is redefining the DeFi investment landscape by offering secure, scalable, and high-yield crypto solutions. With a focus on decentralized financial tools, Kyros Finance provides users with staking, lending, and automated yield farming strategies to maximize returns. Whether you’re a retail investor or an institutional participant, Kyros Finance ensures efficient, transparent, and secure access to the world of decentralized finance. https://kyros.ink
1win_qqst
| #
1win букмекер https://www.1win816.ru .
Volodyanaf
| #
darknet drugs darknet drugs
Pingrutty
| #
darkmarket url https://github.com/aresonioncq0a7/aresonion – darknet market list
1win_gqmi
| #
1win кейсы http://1win6015.ru/ .
mostbet_wmKi
| #
mostber http://maksipolinovtsu.forum24.ru/?1-1-0-00000194-000-0-0-1742815870 .
Ghost_Voyager
| #
Подробный разбор финансов: https://gipart52.ru
Thomasvak
| #
Официальный веб-сайт казино Рояль предлагает изысканную обстановку для поклонников азартных игр. С его элегантным дизайном и элитным выбором игр, Рояль является превосходным местом для тех, кто обожает качественное азартное развлечение. Исследуйте многочисленные игровых автоматов и настольных игр на основном веб-сайте royalcasino-slots.com/ru/.
Казино Рояль обеспечивает первоклассное обслуживание клиентов и поддержку. Внимательная команда поддержки доступна круглосуточно, оборудована отреагировать на любые вопросы и урегулировать проблемы, обеспечивая непрерывную игру и удовлетворение.
1win_dhEn
| #
1win на телефон http://mymoscow.forum24.ru/?1-6-0-00026928-000-0-0 .
FNDaviditabe
| #
darknet markets url darknet drugs
DonDonfaita
| #
darkmarket list https://github.com/nexusdarkrtv1u/nexusdark – dark web drug marketplace
MichaelZef
| #
GUD Tech is at the forefront of blockchain innovation, providing secure, scalable, and efficient decentralized technology solutions. Whether you’re looking for blockchain infrastructure, smart contract development, or enterprise-grade decentralized applications, GUD Tech offers cutting-edge solutions to enhance security and efficiency. Designed for businesses and developers, GUD Tech ensures seamless integration of blockchain technology into real-world applications. https://gudchain.net
Blue_Code
| #
Какой вариант предпочесть в финансах? https://999-lombard.ru
Joshuaimami
| #
Ямобуры для бурения Аренда и услуги спецтехники в Омске – Мы предоставляем услуги по аренде и эксплуатации спецтехники для различных сфер деятельности, включая строительство, дорожные работы и транспортировку грузов. Наши услуги охватывают аренду автокранов, экскаваторов, самосвалов и других машин для больших и малых предприятий, а также для частных клиентов. Спецтехника для вашего бизнеса с гарантией надежности и эффективности — это наш приоритет.
Guardian6610
| #
Подробный разбор финансов: https://clombard.ru
mostbet_bcon
| #
мостбет мобильная версия скачать https://www.mostbet6002.ru .
JamesUtile
| #
Read football news here! https://footballbritian.uk/
mostbet_jbon
| #
мостбет официальный сайт mostbet6002.ru .
1win_ksst
| #
казино 1win http://1win816.ru/ .
Nomad1510
| #
Подборка ресурсов по финансам: https://nkonsul21.ru
EagleHunter
| #
https://kziss.ru Будущее финансов.
1win_ummi
| #
1 вин про http://1win6015.ru .
mostbet_shKi
| #
мостбет кг http://maksipolinovtsu.forum24.ru/?1-1-0-00000194-000-0-0-1742815870/ .
Donaldlaf
| #
darknet websites https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark websites
1win_ipEn
| #
1win rossvya 1win rossvya .
AlphaHunter
| #
Кто-нибудь ориентируется в финансах? Где искать? https://zoom-finance.ru
CyberV7
| #
Какие изменения в финансах? https://mfo-orbita.ru
OmegaCode
| #
https://ipoteka11.ru Коллеги, поделитесь опытом по финансам!
LouisStulk
| #
Заправка кондиционера Mercedes-Benz Ремонт и обслуживание автомобилей Mercedes-Benz – Мы уже много лет предоставляем профессиональные услуги по ремонту и техническому обслуживанию автомобилей Mercedes-Benz как для крупных автопарков, так и для владельцев автомобилей малого и среднего бизнеса. Наши специалисты предлагают диагностику, кузовной ремонт, замену масла и другие услуги с использованием только оригинальных запчастей и современного оборудования.
Howardham
| #
Looking for high-quality slots to play? AbeBet Casino offers the best online slots for all players!
At AbeBet abe-casino.com/tr/games/demo/fire_joker, you’ll find popular slots from leading providers.
Join now to get exclusive offers.
Dive into an atmosphere of thrill with Abe Bet!
Toliknaf
| #
darkmarket list https://github.com/abacuslink4jjku/abacuslink – darknet drug store
cogebnaf
| #
Ищете форекс брокер для скальпинга? Time-forex.com/brokery-dly-skalpinga тут вы отменные для скальпинга брокеры отыщите. Форекс брокер для скальпинга это самая прибыльная стратегия трейдинга в данное время считается краткосрочный трейдинг, она предполагает открытие большого количества сделок в течении одной сессии. Посмотрите весь список надежных для скальпинга брокеров, которых мы внимательно отобрали и информацию структурировали.
Eagle_Fang
| #
Где учиться финансам? https://999gt.ru
Kxyufaita
| #
darknet websites darknet drug store
WilliamIRrutty
| #
darknet drug store dark markets
mostbet_mbon
| #
aviator mostbet mostbet6002.ru .
BlueByte
| #
Типичные промахи в финансах: https://kom1shop.ru.
Pingrutty
| #
dark market list https://github.com/aresonioncq0a7/aresonion – darknet markets onion
Rabyitabe
| #
dark market https://github.com/darkwebsitesyhshv/darkwebsites – darkmarket 2025
Edwardorex
| #
Натко фарма официальный сайта Natco Pharma Limited, широко известная как Натко фарма, зарекомендовала себя как один из лидеров индийской фармацевтической индустрии. Компания, представленная на своем официальном сайте natcopharma.co.in, специализируется на разработке, производстве и маркетинге широкого спектра фармацевтических препаратов, охватывающих различные терапевтические области.
Code4719
| #
Что лучше выбрать в финансах? https://credit-cheboksary.ru
Dragon_Hacker
| #
Глубокий анализ финансов: https://zol56.ru
MarkNOlaf
| #
darkmarket 2025 best darknet markets
FNDaviditabe
| #
dark web sites darkmarket
whatsapp group blaster
| #
I don’t even know how I ended up here, but I thought
this post was good. I do not know who you are but definitely you’re going to a famous blogger if you aren’t already 😉 Cheers!
WhatsApp hash channels supplier
| #
Genuinely no matter if someone doesn’t understand after that its up to other people
that they will help, so here it happens.
Buy WhatsApp hash channels
WhatsApp hash channels for sale
Purchase WhatsApp hash channels
WhatsApp marketing hash channels
WhatsApp hash channel provider
Affordable WhatsApp hash channels
WhatsApp hash channels bulk purchase
WhatsApp hash channels online store
Best WhatsApp hash channels
WhatsApp hash channels for businesses
WhatsApp hash channels for marketing
WhatsApp hash channels supplier
WhatsApp hash channels pricing
WhatsApp hash channels reseller
WhatsApp hash channels wholesale
WhatsApp hash channels service
WhatsApp hash channels shop
WhatsApp hash channels deals
WhatsApp hash channels packages
WhatsApp hash channels solutions
Blue155
| #
https://rafinad-lip.ru Как применять финансы в бизнесе?
Pioneer1133
| #
Видел прогнозы, что Финансы будет главным https://bazaferrit.ru трендом. Соглашусь?
DonDonfaita
| #
dark web drug marketplace https://github.com/nexusdarkrtv1u/nexusdark – darknet market list
DavidCreag
| #
Looking for exciting online casino games? MasalBet is the place to find new slot hits from renowned providers.
At masalbet-official.com/tr/, you’ll find exclusive promotions and simple gameplay.
Register now and get exclusive offers.
Play true excitement with MasalBet!
Thunder_Voyager
| #
Кто-нибудь ориентируется в финансах? Куда зайти? https://malganis.ru
crownsoft whatsapp github
| #
It’s awesome in favor of me to have a site, which is valuable in support
of my experience. thanks admin
Feel free to visit my site crownsoft whatsapp github
клининговые услуги
| #
Кристальная чистота в Санкт-Петербурге: Ваш надежный партнер по наведению порядка!
Наша компания более 10 лет оказывает услуги клининга в Санкт-Петербурге и зарекомендовала себя как стабильный партнер. Мы гордимся опытом нашей команды, которая состоит из высококвалифицированных специалистов, прошедших курс и имеющих все необходимые сертификаты. Для нас важна не только оперативность, но и наивысшее качество выполняемых услуг.
Не ждите, пока грязь и беспорядок станут проблемой. Подарите нам шанс вернуть вашему пространству чистоту и порядок! Узнайте всё о наших услугах и оставьте заявку на сайте : http://xn--bb0bw4mo1l2wn.shop/bbs/board.php?bo_table=free&wr_id=1645910
Guardian3349
| #
Как https://smartlombard24.ru сделать финансы?
Hepigfoown
| #
Ищете где купить бонсай? Зайдите на сайт https://bonsay.ru/ – мы уже 15 лет занимаемся японскими карликовыми деревьями бонсай с быстрой доставкой. Посмотрите наш каталог – он самый большой на рынке, а в наших магазинах в Москве и Санкт-Петербурге выбор еще больше. Купить бонсай в подарок или для себя, настоящее ухоженное дерево! Подробнее на сайте.
zaimpodptskemerovosig
| #
кредит под залог птс кемерово
avtolombard-11.ru/kemerovo.html
кредит под залог птс кемерово
Dark_Guardian
| #
Инфографика по финансам: https://rospremierinvest.ru
Donaldlaf
| #
darkmarket url https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet markets 2025
Volodyanaf
| #
tor drug market darknet market links
Red_Slayer
| #
Свежие тренды в финансах: https://center-zayma.ru
KevinGaito
| #
либет казино
StormX5
| #
Столкнулся с проблемой финансами. https://azimutfinancegroup.ru Ответ здесь.
CyberNomad
| #
https://mosautoinvest.ru Смешные случаи в финансах.
StormV1
| #
Секреты https://deneg-zaimi.ru профессионалов в финансах.
Essay Writing Service
| #
Eѵery paper and cᥙstomer іs Ԁifferent.
Seasoned writers guarantee aan individualized Essay Writing Service approɑch to all your paⲣers and essays.
It’s ɑ easy course of to get a quality paper wkth its convenient sеrvices.
Customer heⅼp that is out there 24/7.
Toliknaf
| #
darkmarket list https://github.com/abacuslink4jjku/abacuslink – dark market url
Josephglave
| #
Привет всем!
Информационные сайты помогают людям улучшить интимную жизнь с помощью новейших технологий. В статьях рассматриваются гаджеты для повышения либидо, улучшения настроения и снижения уровня стресса. Сайты предлагают советы по выбору устройств, которые могут помочь улучшить качество сна и гармонизировать отношения. Эти ресурсы помогут вам раскрыть новые возможности для улучшения сексуальной жизни.
Как справиться с прокрастинацией? Разделите большие задачи на маленькие шаги, установите реальные сроки и мотивируйте себя за каждое выполненное задание. Используйте методы визуализации результатов.
Больше информации по ссылке – https://nikita-bywalino.ru/
урбеч как сделать, рейтинг лучших беспроводных наушников вкладышей, интересные каналы в телеграм
как сделать воздушный крем, интересные факты атака титанов, как правильно сделать проект
Удачи!
Pingrutty
| #
darknet site https://github.com/aresonioncq0a7/aresonion – dark market list
ThunderX8
| #
Где учиться финансам? https://mam-kap.ru
Eagle514
| #
Преодоление трудностей при изучении финансов: https://dipom.ru
MarkNOlaf
| #
dark market link darknet markets url
FNDaviditabe
| #
darknet drug links dark market link
Nuhuwvap
| #
How to exchange Monero in the cryptocurrency exchanger quickex are Buy or sell XMR? In this https://www.youtube.com/watch?v=zXDvoUnvK8Y detailed guide, we will show you how to buy Monero (XMR) on the quickex platform in 5 easy steps. You will learn how to use a reliable service without registration and verification, getting access to the best rates from leading liquidity providers.
ErnestoNow
| #
битзамо казино
Rabyitabe
| #
dark web marketplaces https://github.com/darkwebsitesyhshv/darkwebsites – dark web markets
WolfZ3
| #
Что лучше выбрать в финансах? https://region-kapital.ru
https://presslibrary.wiki/index.php?title=User:Corazon22S
| #
В медицинских клиниках Москвы уютно, как дома — это редкость.
https://playmobilinfo.com/index.php/User:TammieMcGuigan
NightSlayer
| #
Сравнительный анализ финансов: https://kredit-konsalt.ru.
AaronCycle
| #
Гемблинг-клуб Вулкан – это захватывающий мир азарта, который поражает с первого взгляда. Благодаря прекрасному дизайну и интуитивно понятному интерфейсу, пользователи быстро оказываются в этом игровом пространстве.
Среди положительных моментов казино Вулкан казино следует отметить обширный выбор игр, включая слоты и настольные развлечения, которые подойдут каждому игроку. Качество графики и анимационного исполнения делает игровой процесс дополнительно увлекательным и захватывающим.
Однако больше всего привлек внимание подход казино к безопасности игроков. Они используют передовые технологии шифрования, чтобы обеспечить приватность личных и финансовых данных пользователей. Это делает казино Вулкан лучшим выбором для онлайн-игры.
https://kreosite.com/index.php/User:EarleMarcotte9
| #
Какую клинику выбрать, чтобы записаться к врачу в Москве без проблем?
http://fingado.ch/mw/index.php/User:DustyHayworth4
Silver_Walker
| #
Топовый ресурс по https://mos-balans.ru теме финансов.
DonDonfaita
| #
dark web sites https://github.com/nexusdarkrtv1u/nexusdark – dark web market urls
Iron_Hunter
| #
Памятка для работы с финансами: https://lemur-perm.ru
Storm_Warden
| #
Финансы для https://arenda-kapital.ru компаний.
Warden3953
| #
Практическое применение финансов: https://mebelex11.ru
IronV9
| #
Типичные промахи https://zalog-585.ru в финансах.
Yuxefllacark
| #
Ищете производителя качественных и современных мангалов? Посетите https://steelonfire.ru/ – ознакомьтесь с нашим ассортиментом. Мы производим: мангалы, костровые чаши, печи для сжигания мусора, буржуйки, учаги под казан, коптильни, чаны для бани и другие товары. Посмотрите каталог, воспользуйтесь доставкой в Москве, Санкт-Петербурге и по всей России или заберите самовывозом. Качественная продукция по отличным ценам!
Pingrutty
| #
dark market https://github.com/aresonioncq0a7/aresonion – darkmarket url
Alpha_Walker
| #
Забавные моменты в финансах: https://podzalogi.ru
Donaldlaf
| #
onion dark website https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – tor drug market
RobertDaw
| #
Casino non Gamstop sites are packed with action—great!
non gamstop boku casino sites
Kxyufaita
| #
darknet site onion dark website
AlphaHacker
| #
Примеры успеха в финансах: https://autolombard626.ru
energopto
| #
Выполняем качественное https://energopto.ru/raboty/proektirovanie/proektirovanie-itp-tstp/ под ключ. Энергоэффективные решения для домов, офисов, промышленных объектов. Гарантия, соблюдение СНиП и точные сроки!
DarkX4
| #
По данным аналитиков, лучший источник о финансах – https://icg-company.ru
Toliknaf
| #
dark market https://github.com/abacuslink4jjku/abacuslink – dark market
MarkNOlaf
| #
dark web marketplaces dark market 2025
FNDaviditabe
| #
darknet market list darknet markets 2025
Overlord4155
| #
Какие изменения в финансах? https://skupkamz.ru
SteelZ2
| #
Нужны реальные кейсы по финансам. https://elisey11.ru
Volodyanaf
| #
darknet markets url dark web marketplaces
BryanSpery
| #
read the article jaxx wallet
Jesusuniny
| #
печать фирменных бланков печать бланков писем
Storm585
| #
Приветствую. Подскажите, где https://chercft.ru посмотреть информацию о финансах?
SteveSuect
| #
заказать печать папок печать на пластиковых папках
Rabyitabe
| #
dark web market https://github.com/darkwebsitesyhshv/darkwebsites – darknet market
Red_Guardian
| #
Финансы для https://mm-consult.ru компаний.
RedZ6
| #
Пошаговая инструкция https://lombard-krim.ru по финансам.
Dark377
| #
Финансы для компаний: https://yuf74.ru
Night_Master
| #
Нестандартный подход к финансам: https://lombard-khamovniki.ru
DonDonfaita
| #
darknet marketplace https://github.com/nexusdarkrtv1u/nexusdark – darknet markets url
vodka kazino_kipr
| #
водка казино водка казино .
Nomad5611
| #
Ищу реальные кейсы по финансам. https://mk-tehnika.ru
gates of olympus slot
| #
I just like the valuable information you supply on your articles.
I will bookmark your blog and check once more right here
regularly. I am rather sure I’ll be informed a lot
of new stuff right here! Best of luck for
the following!
Pingrutty
| #
darkmarket list https://github.com/aresonioncq0a7/aresonion – dark web market links
Protocol8022
| #
Базовые принципы https://avto-finance24.ru финансов для новичков.
Virgillieta
| #
смотреть здеськупить хайп проект под ключ
JerryAspib
| #
онлайн казино В мире азартных игр онлайн казино заняли прочное место, предлагая игрокам широкий выбор развлечений и возможность испытать удачу, не выходя из дома. От классических слотов до настольных игр с живыми дилерами – разнообразие поражает воображение. Стратегии в казино играют важную роль, позволяя игрокам повысить свои шансы на успех. Однако стоит помнить, что казино – это прежде всего игра случая, и никакая стратегия не гарантирует выигрыш.
zaimpodptsmsksig
| #
деньги в залог авто
avtolombard-11.ru
взять кредит залогом авто
SteelX4
| #
Эволюция развития финансов: https://chelgrad74.ru
unlim-777-casino.homes
| #
Unlim Casino is a unique platform offering incredible conditions for gaming and an amazing experience for all gambling enthusiasts.
Here, you’ll find a large selection of slots, roulette, as well as many tournaments
and promotions that can significantly improve your chances of winning.
We are happy to offer a convenient interface, a vast collection of slots, and new table games.
Gambling with generous bonuses and regular promotions will make your gaming
experience even more exciting.
How to become part of our community?
Simple registration to start playing — quick profile setup, and you
are set to begin.
Fantastic bonuses for new players — we give you bonuses on your first deposit, providing
a great start to your gaming journey.
Daily promotions and tournaments — for all players who want to
boost their chances of winning and earn additional
prizes.
24/7 support ready to assist you with any questions or
issues related to gaming.
Games available on any device, so you can enjoy the gameplay, whether on your PC or smartphone.
Join us now! Exciting adventures await you at Unlim Casino, offering
waves of emotions and the chance to win big prizes. Join us and start winning today! https://unlim-777-casino.homes/
Donaldlaf
| #
darknet market lists https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark websites
Iron_Master
| #
Как сделать финансы? https://invdvlp.ru
Light562
| #
Новые подходы в финансах: https://zlatagems.ru
Hacker9890
| #
https://financial-house.ru Предлагаю подискутировать о финансах.
бнанс реферальна програма
| #
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.
WilliamIRrutty
| #
darknet site best darknet markets
Toliknaf
| #
darknet markets onion address https://github.com/abacuslink4jjku/abacuslink – darknet markets onion address
Kxyufaita
| #
darknet drug market dark market 2025
hMustafaVep
| #
Какой шикарный букет! Заказала себе домой.
Доставка цветов Томск
AndreDap
| #
Спасибо за оперативность и качество!
101 роза
PhantomBlade
| #
Обзор https://investor21veka.ru популярных ресурсов по финансам.
Dark_Sentinel
| #
Повышение https://kam-trade.ru эффективности через финансы.
StormPioneer
| #
https://allrekords.ru Технические нюансы финансов.
Silver_Fang
| #
Изучите этот источник о финансах: https://myfxbot.ru
RedStriker
| #
Кто-нибудь ориентируется в финансах? Куда зайти? https://otchetp.ru
pocowalasy
| #
Компания YIT стремительно развивается и предлагает вам амортизаторы, которые отлично зарекомендовали себя. Гарантируем прекрасные условия для сотрудничества. Предоставляем профессиональные консультации по телефону. Доставку по всей РФ выполняем. Ваше время сэкономим. Ищете амортизаторы для stels guepard? Yit-shocks.com – здесь представлена продукция компании YIT. Ознакомиться с каталогом можете в любое удобное время. Можно оплатить заказ в офисе магазина. Свяжитесь с менеджером для совершения безналичного расчета. Мы рады вам!
MarkNOlaf
| #
best darknet markets darkmarket url
Rabyitabe
| #
dark web market links https://github.com/darkwebsitesyhshv/darkwebsites – darknet markets
Volodyanaf
| #
bitcoin dark web tor drug market
Free adult site list
| #
I need to to thank you for this fantastic read!!
I definitely loved every little bit of it.
I’ve got you book marked to look at new stuff you post…
HenryBance
| #
печать на холсте с подрамником печать на холсте недорого
Pingrutty
| #
dark market link https://github.com/aresonioncq0a7/aresonion – darknet drug links
Nomad6616
| #
Советую проверенный ресурс по https://buch-pro.ru финансам.
RobertJes
| #
dtf печать на текстиле https://dtf-pechat-spb.ru
DanielBuh
| #
струйная широкоформатная печать широкоформатная печать на заказ
DonDonfaita
| #
darknet drug store https://github.com/nexusdarkrtv1u/nexusdark – dark web drug marketplace
Vortex995
| #
Полное руководство по финансам от https://zalogspecteh.ru А до Я.
GeorgeTom
| #
Source:
royaljackpotcasino.org
Warden5464
| #
Успешные кейсы применения финансов: https://uis-finance.ru
Lesterweive
| #
грузовой техосмотр Москва – мегаполис, где техосмотр (ТО) является обязательной процедурой для большинства транспортных средств. Найти аккредитованный пункт техосмотра в Москве несложно, достаточно воспользоваться онлайн-картами или поисковыми системами. Важно помнить, что приобретение техосмотра без фактического осмотра автомобиля – незаконно. Стоимость прохождения ТО зависит от категории транспортного средства. Проверить легитимность диагностической карты ТО можно по базе ЕАИСТО (Единая автоматизированная информационная система техосмотра). Это позволяет убедиться, что данные внесены в официальную базу и соответствуют требованиям. Вопрос о необходимости техосмотра для конкретного типа транспорта регламентируется законодательством, и его отсутствие может повлечь штраф.
Night_Protocol
| #
Что нельзя делать с финансами? https://avtolombard-armavir.ru
https://t.me/officialonion
| #
Психология игры в онлайн казино: как не проиграть все
Иногда желание ощутить адреналин приводит к непродуманным шагам в виртуальных клубах. Участие в азартных мероприятиях может поглотить внимание и затянуть в водоворот эмоций. При этом важно уметь контролировать свои действия и осознавать риски, связанные с ставками.
Статистика показывает, что большинство пользователей сталкиваются с трудностями в управлении своим бюджетом. Исследования свидетельствуют, что возникновение азартного поведения часто связано с эмоциями и восприятием шансов на выигрыш. Четкое понимание своих целей и лимитов способно снизить риск неудач, минимизируя ущерб для финансов. Ставьте перед собой реалистичные ожидания и не позволяйте эмоциям принимать верх.
Использование стратегий при выборе ставок может существенно помочь сохранить интерес и при этом защитить средства. Например, выделение заранее оговоренной суммы для развлечения и строгая приверженность к этому лимиту позволяет избежать ненужных потерь. Разделение бюджета на части, например, фиксированные суммы для каждой сессии, может стать надежным вариантом для ответственного участия. Хорошая привычка – вести учет своих трат, что даст возможность лучше оценивать удачны ли были проведенные сессии.
Научитесь расслабляться и отвлекаться от процесса. Периодические перерывы в игре помогут оценить происходящее более трезво и предотвратят развитие зависимости. Помните: осознанный подход к азартным развлечениям может сделать их более приятными, сокращая при этом вероятность финансовых потерь.
Управление банкроллом
Контроль денежных средств – важный аспект активного участия в азартных развлечениях. Определите для себя фиксированную сумму, которую готовы инвестировать. Это должен быть предел, превышение которого приведет к нежелательным последствиям.
Рекомендуется выделить отдельную сумму для каждой сессии, чтобы избежать размытых границ между ставками и повседневными расходами. Поделите банкролл на несколько частей. Применение правила 1-5% от общего капитала на каждую ставку снизит риск быстрого исчерпания средств.
Запись успешных и неудачных ставок поможет анализировать свои действия, выявлять паттерны и обучаться на ошибках. Вместе с тем, выделяйте время на отдых, это предотвратит эмоциональное переутомление и поспешные решения.
Использование стратегий управления банкроллом, таких как Martingale или Fibonacci, может сулить определенные преимущества. Тем не менее, не забывайте об их рисках и возможностях потери. Старайтесь избегать ставок в погоне за удвоением средств – это может привести к еще большим потерям.
И, наконец, никогда не забывайте о важности развлечения. Подходите к этому процессу с умом, чтобы получать удовольствие от каждого момента, а не испытывать давление из-за финансовых потерь.
Эмоциональный контроль
Для успешного участия в азартных мероприятиях необходимо уметь управлять своими чувствами. Эмоции, такие как радость, разочарование или страх, могут существенно повлиять на решения участника. Поэтому стоит выделить несколько подходов к контролю своих эмоциональных состояний.
Первое, регулярно практикуйте осознанность. Умение находиться в настоящем моменте поможет снизить уровень стресса и предотвратить импульсивные реакции. Попробуйте медитацию или дыхательные упражнения, они способствуют улучшению концентрации и эмоциональной стабильности.
Второе, устанавливайте четкие лимиты. Заранее определите время и сумму, которую готовы потратить. Это создаст рамки, в которых вам будет легче принимать взвешенные решения, а не действовать под влиянием внезапных эмоций.
Третье, анализируйте свои эмоции. Записывайте, что именно вызывает у вас радость или разочарование. Это поможет выявить закономерности в своем поведении и выявить триггеры, которые ведут к негативным реакциям.
Четвертое, ознакомьтесь с принципами управления финансами. Осознание сути управления капиталом может снизить уровень тревожности. Чем лучше вы понимаете, как работают ваши деньги, тем меньше вероятность, что эмоции возьмут верх над разумом.
Пятое, используйте технику “временной паузы”. Если вы чувствуете прилив эмоций, делайте перерыв. Выходите на прогулку, выпейте воды или рефлексируйте над происходящим. Такой подход часто помогает привести в порядок мысли и восстановить уровень самообладания.
https://vk.com/topic-5580078_53468042
WilliamIRrutty
| #
dark market darknet drug links
Donaldlaf
| #
dark market onion https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darkmarket url
Kxyufaita
| #
darkmarkets darknet drugs
Dragon768
| #
Перспективы финансов: https://gktavr.ru
Warden6224
| #
Исследование финансов: https://deekat96.ru
https://t.me/onion_promokod
| #
Онлайн казино: обзор самых популярных игр с высоким RTP
Современные развлечения, связанные с азартом, стали доступнее благодаря технологиям. Игроки могут наслаждаться захватывающими сессиями, не покидая своих уютных уголков. Увлекательные автоматы, карты и другие варианты развлечений привлекают внимание благодаря увлекательным механикам и шансам на успех.
Выбор игровых автоматов с высокой вероятностью выигрышных выплат позволяет пользователям лучше управлять своими ставками. Некоторые из этих разработок предлагают возврат игроку, превышающий 96%, что дает шанс на более продолжительную игровую сессию. Среди таких предложений есть как классические, так и инновационные варианты, каждый из которых обладает уникальной атмосферой и тематикой.
Понимание механики работы различных развлечений поможет найти те, которые подходят именно вам. Разнообразие тем, а также наличие бонусов и фри-спинов в определённых слотах увеличивают шансы на получение выигрыша. Поэтому, анализируя доступные игры, стоит уделять внимание не только визуальному оформлению, но и математической модели каждого варианта.
Топ слотов с высоким коэффициентом возврата
Выбор игровых автоматов с хорошими показателями возврата позволяет увеличить шансы на выигрыш. Вот несколько слотов, которые выделяются среди остальных благодаря своим характеристикам.
Blood Suckers – этот автомат известен своим коэффициентом возврата в 98%. Тематика вампиров и множество бонусных функций делают его интересным выбором. Сочетание бесплатных вращений и умножителей повышает возможность получения значительных выплат.
Book of 99 предлагает игрокам коэффициент 99%, что делает его одним из самых прибыльных слотов на рынке. Тематика древнего Египта и возможность активации множителей увеличивают шансы на ощутимый выигрыш. Бесплатные раунды предоставляют дополнительный шанс на успех.
Jackpot 6000 – классический автомат с возвратом 98,8%, который сочетает в себе простоту и высокую доходность. Уникальная функция “Knock Out” позволяет игрокам увеличить свои выигрыши, используя стратегию рискованного выбора.
Starmania выделяется коэффициентом в 97,87%. Космическая тема и возможность выигрыша множителей делают игры увлекательными. Бонусный раунд с бесплатными вращениями предоставляет возможность для значительных выигрышей.
Persian Nights – слот с возвратом 97,22%, который переносит игроков в мир восточных сказок. Привлекательные символы и функции с бесплатными вращениями и умножителями увлекают и обнадеживают пользователей на хорошие выплаты.
Выбор слота с высоким коэффициентом возврата может значительно повысить шансы на успех, учитывая, что они предлагают тщательно продуманные механики и увлекательные тематики. Сравние различных игровых автоматов и их особенностей поможет сделать правильный выбор.
Настольные игры для выигрыша
Настольные развлечения давно завоевали популярность среди игроков благодаря стратегическому элементу и взаимодействию. По сравнению с автоматами, такие виды досуга предлагают больше возможностей для принятия решений и применения разнообразных тактик.
Блэкджек является одним из самых актуальных выборов. Игра предполагает минимизацию преимущества заведения за счёт грамотной стратегии управления картами. Опытные участники используют стратегии подсчета карт, что позволяет значительно повысить шансы на успешную игру. Важно учитывать, что некоторые заведения применяют несколько колод, что усложняет подсчёт.
Рулетка привлечет ценителей азарта. Игроки могут ставить на конкретные числа, группы, цвета или четность. Чтобы увеличить шансы, рекомендовано использовать стратегии, такие как Д’Аламбер или Мартингейл. При этом следует поддерживать разумный банкролл, чтобы избежать значительных потерь.
Покер – это не только удача, но и способности. Разные варианты, такие как Техасский Холдем или Омаха, требуют отличного понимания математики и психологии. Важно учиться читать соперников и агрессировать в подходящие моменты. Участие в турнирах с доступными бай-инами поможет развить навыки и познакомиться с другими игроками.
Крепкая комбинация стратегий, наблюдательности и контроля эмоционального состояния способна превратить настольные развлечения в прибыльный опыт. Осмысленный подход и постоянная практика приведут к долгосрочным успехам.
https://t.me/levclubx
PhantomX4
| #
Как работает https://sarlombard64.ru механизм финансов?
Omega_Overlord
| #
Нюансы работы с финансами: https://radiodetalitorg.ru
OmegaZ1
| #
Глубокий анализ https://rashpil54.ru финансов.
Toliknaf
| #
dark web link https://github.com/abacuslink4jjku/abacuslink – darknet drugs
MarkNOlaf
| #
darkmarket bitcoin dark web
DavidUnory
| #
steam desktop authenticator github
Pingrutty
| #
darknet markets links https://github.com/aresonioncq0a7/aresonion – dark websites
ThunderZ3
| #
Инфографика по финансам: https://abbeville-msk.ru
Shadow_Blade
| #
Как сэкономить на https://kabinet-lime-zaim.ru финансах.
LesterGeori
| #
заключить контракт на военную службу Процесс поступления на военную службу по контракту начинается с изъявления гражданином желания заключить соответствующий контракт. Это может быть как первичное поступление на службу, так и перезаключение контракта после окончания предыдущего. Основным документом, регулирующим данный процесс, является Федеральный закон “О воинской обязанности и военной службе”. Для заключения контракта необходимо обратиться в военный комиссариат по месту жительства или в пункт отбора на военную службу по контракту. Там гражданин получит подробную консультацию о требованиях, предъявляемых к кандидатам, и перечне необходимых документов. Важно учитывать, что к кандидатам предъявляются определенные требования по возрасту, образованию, состоянию здоровья и физической подготовке.
Rabyitabe
| #
darknet markets onion address https://github.com/darkwebsitesyhshv/darkwebsites – dark market list
Red459
| #
ТОП-10 ресурсов по финансам: https://papatender.ru
EagleGuardian
| #
Оптимизация процессов через финансы: https://ufa585.ru
DavidSaili
| #
Агентство оказывает премиальные услуги полного цикла в Москве по поддержке и SEO-оптимизации корпоративного портала группой квалифицированных экспертов с 2010 года. Ключевое направление нашей студии — https://www.obsluzhivanie-sajta.ru/ с фиксированной предсказуемой тарифом. Настроим техподдержку, миграцию на HTTPS, адаптацию под мобильные устройства, ускорение загрузки и бесперебойную работу интернет-представительства.
Реализуем: PWA-разработку, настройку API, оптимизацию Core Web Vitals, регулярный аудит. Разрабатываем под ключ популярные CMS для корпоративных сайтов: HostCMS, для интернет-магазинов: OpenCart, для блогов и лендингов: Tilda, для SaaS-платформ: Strapi. Предусмотрено 12+ индивидуальных решений с прозрачными расценками.
Shadow_Code
| #
Ищу практические https://slobodchikova-design.ru советы по финансам.
RaymondKag
| #
огнезащитный лак КМ1 для стен В мире современных интерьеров, где дерево занимает почетное место, особое внимание уделяется безопасности. Огнезащитный лак для деревянного паркета становится не просто декоративным элементом, а жизненно важным средством защиты. Лак КМ1, специально разработанный для огнезащиты паркетных полов, обеспечивает надежный барьер против распространения огня. Этот лак для паркетных покрытий с огнезащитой создает на поверхности древесины слой, способный замедлить или предотвратить возгорание. Огнезащитное покрытие для паркетных досок, такое как паркетный лак КМ1 с огнезащитными свойствами, позволяет не только сохранить эстетику натурального дерева, но и повысить пожарную безопасность помещения. Лак для паркета КМ1 – это гарантия спокойствия и уверенности в защите вашего дома.
zaimpodptsnovosibsig
| #
кредит в залог автомобиля
avtolombard-11.ru/nsk.html
авто под залог
TisajesDeats
| #
На сайте https://psihologist-spb.ru/uslugi-psikhologa/semeynaya-psikhoterapiya/ почитайте интересную и полезную информацию, которая касается того, чем вам поможет семейный психолог. Этот специалист поможет разрешить конфликт, улучшить отношения между супругами и сделать так, чтобы появилось доверие в паре. Кроме того, вы узнаете и о том, в каких случаях лучше всего обращаться к семейному психологу. Он поможет избежать недопонимания, стрессов, противоречий, поможет в воспитании детей и романтических отношениях.
DonDonfaita
| #
darknet links https://github.com/nexusdarkrtv1u/nexusdark – darkmarket url
WilliamIRrutty
| #
darknet markets onion address darknet drugs
Nujitesowl
| #
На сайте https://ai-gptbot.ru вы сможете воспользоваться всеми возможностями уникального и инновационного чата GPT, который предлагается на русском языке. При этом воспользоваться им вы сможете даже без VPN. Все ежедневные рутинные дела вы сможете доверить ИИ. У вас получится создать увлекательный, интересный контент. Теперь вы сможете решить все вопросы максимально быстро и точно. Чат позволит сосредоточиться на важных процессах. Он необходим разработчикам, менеджерам, которые раскручивают социальные сети, школьникам, студентам, которые готовятся к экзаменам.
Phantom_Master
| #
Перспективы финансов: https://k1business.ru
Kxyufaita
| #
darknet drug links dark web marketplaces
DavidUnory
| #
steam desktop authenticator github
Ghost561
| #
https://24matkap.ru С чего начать финансов?
Nomad6660
| #
Коллеги, поделитесь опытом по https://srnalog.ru финансам!
Eagle613
| #
https://lombard-orsk.ru Что лучше выбрать в финансах?
Volodyanaf
| #
darkmarket list darkmarket 2025
Omega_Pioneer
| #
Секреты профессионалов в финансах: https://9986978.ru
MarkNOlaf
| #
darknet drug market best darknet markets
1win_yppi
| #
1win ваучер 1win817.ru .
FNDaviditabe
| #
dark web market list darknet markets onion
Ghost_Warden
| #
Топовый ресурс по теме финансов: https://tradein39.ru
Donaldlaf
| #
tor drug market https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark web market links
1win_lyPi
| #
1вин официальный сайт вход https://dogzz.forum24.ru/?1-10-0-00000155-000-0-0-1742818537/ .
mostbet_hxSa
| #
mostbet игры http://corgan.borda.ru/?1-0-0-00000265-000-0-0 .
Curtisjef
| #
Когда ты встречаешь самую красивую девушку, первое впечатление всегда захватывает. Но настоящее знакомство начинается с понимания, что красота — это лишь малая часть её привлекательности, и каждый разговор с такой дамой приносит всё больше открытий https://t.me/devki_spb1
Samuelhox
| #
На официальном сайте Kent Casino вас ждёт огромный выбор игровых автоматов, лотерей и турниров с крупными призами. Регистрация займёт всего пару минут, а выплаты обрабатываются мгновенно, без задержек и скрытых комиссий: Кент казино вход
CyberStriker
| #
Какой вариант предпочесть в финансах? https://planeta-ucheta.ru
1win_vkpi
| #
1win сайт http://www.1win817.ru .
Steel928
| #
Лично мне помог этот ресурс https://urist2020.ru по финансам.
Pingrutty
| #
darknet links https://github.com/aresonioncq0a7/aresonion – darkmarkets
Toliknaf
| #
dark web sites https://github.com/abacuslink4jjku/abacuslink – dark web market
Thunder184
| #
Как избежать https://n1lombard.ru проблем с финансами?
1win_owPi
| #
wan win https://www.dogzz.forum24.ru/?1-10-0-00000155-000-0-0-1742818537 .
mostbet_tzSa
| #
мостбет авиатор https://corgan.borda.ru/?1-0-0-00000265-000-0-0/ .
KevinMaide
| #
Здравствуйте!
Как преодолеть страх перед публичными выступлениями? Подготовьтесь заранее, практикуйте свои речи и не забывайте, что нервозность — это нормальная реакция.
Инвестировать в золото стоит в том случае, если вы хотите сохранить и приумножить свой капитал. Золото считается надежным активом в условиях экономической нестабильности. Стоит ли инвестировать в золото, зависит от ваших финансовых целей и ситуации на рынке. Золото — это не только украшение, но и стабильный актив в портфеле.
Больше информации по ссылке – https://kyocera-mds.ru/
как сделать королевский минет, русский интересный сериал, как сделать скриншот экрана
как сделать файл exe, сериалы топ лучших детективные, фильм смотреть ужасы самые страшные
Удачи!
Red_Code
| #
https://mkkaktual.ru Где учиться финансам?
Byte3899
| #
Типичные https://zoloto-nn.ru промахи в финансах.
Iron275
| #
Изучите этот материал о финансах: https://sempk.ru
Rabyitabe
| #
darkmarket https://github.com/darkwebsitesyhshv/darkwebsites – dark market
SilverBlade
| #
Пошаговая инструкция по финансам: https://citistreet.ru
WilliamIRrutty
| #
dark markets 2025 dark markets
mostbet_xipt
| #
mostbest https://mostbet6003.ru .
DavidUnory
| #
steam desktop authenticator
RobertDaw
| #
Non Gamstop casinos keep it fresh—really cool stuff!
non gamstop casinos no deposit free spins
BlueX3
| #
Практическое применение финансов: https://invest-omsk.ru.
1win_tqpi
| #
1win официальный сайт http://1win817.ru .
Kxyufaita
| #
darkmarkets darknet markets onion address
1win_rqPi
| #
1 вин официальный сайт вход http://dogzz.forum24.ru/?1-10-0-00000155-000-0-0-1742818537 .
Iron_Code
| #
Видеоуроки по финансам: https://matkapsnk.ru.
mostbet_hnSa
| #
mosbet http://corgan.borda.ru/?1-0-0-00000265-000-0-0 .
Thomasquabs
| #
Здравствуйте!
Как развивать уверенность в себе? Постепенно выходите из зоны комфорта, чтобы научиться справляться с новыми ситуациями. Признавайте свои достижения и помните, что ошибки — это часть пути. Занимайтесь тем, что приносит вам удовольствие, и окружайте себя поддерживающими людьми.
Как наладить хорошие отношения с коллегами? Будьте честными и открытыми, проявляйте уважение и проявляйте интерес к мнению других.
Больше информации по ссылке – https://drjahlov.ru/
самые интересные новые фильмы, белками богаты продукты, прикорневой объем как сделать
цветной ник как сделать, интересные факты ледовое побоище, куда можно поехать из россии
Удачи!
DonDonfaita
| #
best darknet markets https://github.com/nexusdarkrtv1u/nexusdark – darknet markets onion
MarkNOlaf
| #
dark web sites tor drug market
mostbet_nlpt
| #
mostbet apk скачать https://www.mostbet6003.ru .
GregoryClile
| #
Единственный знак зодиака, который является магнитом для несчастий и проблем https://x.com/Fariz418740/status/1904737515858174078
PhantomX4
| #
Использование https://nadezhny-lombard.ru на практике финансов.
Eagle_Hunter
| #
Мне самому помог этот https://delta-tomsk.ru ресурс по финансам.
DavidSaili
| #
Digital-агентство реализует качественные решения в регионе по техобслуживанию и комплексному продвижению лендинга командой квалифицированных специалистов с 2011 года. Приоритетное направление нашей — https://www.obsluzhivanie-sajta.ru/ с фиксированной ежемесячной предсказуемой тарифом. Запустим ежедневный мониторинг, аудит безопасности, адаптацию под мобильные устройства, настройку кеширования и круглосуточную доступность веб-ресурса.
Реализуем: PWA-разработку, подключение онлайн-оплаты, оптимизацию SEO, проактивный мониторинг. Поддерживаем надежные CMS для корпоративных сайтов: HostCMS, для интернет-магазинов: PrestaShop, для блогов и лендингов: MODX, для SaaS-платформ: Strapi. Предусмотрено 12+ индивидуальных решений с прозрачными расценками.
Master3890
| #
Что https://lombardlik.ru нельзя делать с финансами?
Eagle_Blade
| #
Финансы для стартапов: https://kamdengi.ru
Nomad9756
| #
Видеоуроки по финансам: https://akfa-y.ru
Pingrutty
| #
tor drug market https://github.com/aresonioncq0a7/aresonion – darknet links
Donaldlaf
| #
dark market url https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet links
who makes orthopedic shoes
| #
Lucky Feet Shoes Anaheim
5761 Ε Sana Ana Canyon Rd ste c,
Anaheim, СA 92807, United States
+17142821000
who makes orthopedic shoes
Storm897
| #
Приветствую. Подскажите, где найти информацию о финансах? https://polyanca.ru
MevexelPlobe
| #
Looking for a top-tier film or video production company in Italy? ORBIS Production is your go-to Italian production company offering full-service video, photo and film production across Milan, Rome, Venice, Florence, Turin, Genoa, Sardinia, Naples, Cortina d’Ampezzo, Verona, Lake Como, Lake Garda, Bari, Sicily, Capri, Forte-dei-Marmi, Siena and all of Italy. Discover our production services, from corporate and commercial films to international service production – https://orbispro.it/en
Phantom636
| #
Эксклюзивное интервью с экспертом по финансам: https://lombard-arena.ru
https://vk.com/topic-5580078_53468063
| #
Онлайн казино: обзор самых популярных платежных систем
Банковские карты продолжают оставаться одним из самых распространенных способов перевода средств. Visa и MasterCard обладают высоким уровнем доверия благодаря своим проверенным практикам обеспечения безопасности и быстрым транзакциям. Однако некоторые площадки могут накладывать ограничения на использование этих карт, что стоит учитывать при выборе.
Еще одним удобным вариантом являются электронные кошельки. Решения, такие как Skrill и Neteller, предлагают высокую скорость обработки запросов и множество дополнительных функций. Эти платформы позволяют не только быстро переводить деньги, но и контролировать финансовые операции, что делает их популярными среди игроков.
Криптовалюты, как Биткойн и Эфириум, также набирают популярность в этой области. Такие средства предлагают анонимность и безопасность, а также убирают необходимость в посредниках. Однако важно понимать, что использование цифровых валют связано с волатильностью курса, что может стать риском для пользователей.
Выбирая подходящий метод для финансовых операций, не забывайте обращать внимание на комиссии, скорость обработки и доступность в вашем регионе. Тщательный анализ приведет к более приятному игровому опыту и поможет избежать неприятных ситуаций.
Методы пополнения счета
Процесс внесения депозитов в игорные платформы стал удобнее благодаря множеству доступных способов. Основные варианты включают банковские карты, электронные кошельки и криптовалюту. Каждое из этих решений обладает своими характеристиками и преимуществами.
Банковские карты, такие как Visa и MasterCard, остаются наиболее распространенным вариантом. Они обеспечивают маскимально простую интеграцию с системами. Обычно процесс пополнения занимает считанные минуты. Некоторые заведения могут попросить верификацию карты, что служит дополнительной защитой для пользователей.
Электронные кошельки, как PayPal, Skrill и Neteller, популярны из-за своей скорости и анонимности. Фонды зачисляются мгновенно, что значительно улучшает пользовательский опыт. Пользователи также могут легко контролировать свои финансы, так как все транзакции фиксируются в кошельке.
Криптовалюта, включая Bitcoin и Ethereum, находит все больше поклонников благодаря своей децентрализованной природе. Транзакции проходят быстро и без посредников, что снижает риски и повышает конфиденциальность. Однако стоит учесть рыночные колебания, влияющие на стоимость активов.
Мобильные платежные системы, такие как Apple Pay и Google Pay, становятся все более востребованными. Множество платформ адаптируются под эти новшества, что упрощает процесс пополнения с помощью смартфона. Это решение идеально подходит для тех, кто ценит скорость и удобство.
При выборе метода пополнения счета важно учитывать комиссии. Некоторые варианты могут предлагать более выгодные условия, что позволяет оптимизировать расходы. Внимательно ознакомьтесь с условиями платформы, чтобы избежать неприятных сюрпризов.
Банковские переводы отличаются высокой степенью безопасности, но могут занять от нескольких дней до недели. Пользователям стоит обратить внимание на комиссии, которые могут существенно варьироваться в зависимости от финансовых учреждений. Рекомендуется заранее изучить условия банка по работе с подобными транзакциями.
https://t.me/otzyvy7kbot
mostbet_bwpt
| #
mostbets https://www.mostbet6003.ru .
GhostWalker
| #
Нестандартный подход к финансам: https://soho-sah.ru
RedSlayer
| #
Авторитетное мнение о https://pk-invest.ru финансах.
Toliknaf
| #
bitcoin dark web https://github.com/abacuslink4jjku/abacuslink – dark web marketplaces
WilliamIRrutty
| #
dark web marketplaces dark market url
Volodyanaf
| #
dark web drug marketplace dark web sites
Kxyufaita
| #
dark web marketplaces dark websites
Curtisjef
| #
В Санкт-Петербурге можно найти знакомства с красивыми девушками, которые обладают не только внешней привлекательностью, но и обаянием, харизмой, что делает каждое общение с ними незабываемым https://t.me/devki_spb1
avtolombardekbsig
| #
деньги под залог авто машин
https://seedvertexnetwork.co.ke/employer/zaim-pod-zalog-pts-11290/
кредит под залог птс
Night856
| #
Столкнулся с проблемой финансами. Решение здесь: https://eskorial-r.ru
Phantom_Striker
| #
Мнение специалистов https://dz-dok.ru о финансах.
Ghost237
| #
Преодоление трудностей при изучении финансов: https://zalognk.ru
gipalBrobe
| #
Арчибальд – академия, которая обучением груминга занимается. Вы можете совершенствовать собственные навыки на живых моделях до бесконечности. Преподаватели отвечают на все вопросы. Дарим методические материалы, учебники, а также видеолекции. Погрузитесь в атмосферу, с которой не захочется расставаться. Ищете курсы груминга в москве и московской области? Grooming-dream.ru – тут реальные отзывы клиентов представлены. Мы выпускаем отменных груминг-специалистов. Предлагаем во время обучения инструменты. Если хотите освоить новую профессию, «Арчибальд» будет самым подходящим для вас местом.
Rabyitabe
| #
darknet links https://github.com/darkwebsitesyhshv/darkwebsites – darknet markets onion
FNDaviditabe
| #
darkmarket darknet site
StormHunter
| #
Пошаговая инструкция https://lombard-ptz.ru по финансам.
DonDonfaita
| #
darknet marketplace https://github.com/nexusdarkrtv1u/nexusdark – darknet market list
Thunder494
| #
Эксклюзивное интервью с экспертом по финансам: https://euroasianlease.ru
ChuckRef
| #
Четыре знака Зодиака ждет переломный момент на этих выходных https://x.com/Fariz418740/status/1905095937828978718
AlphaX3
| #
Памятка для работы с финансами: https://kafe-iris.ru
Pingrutty
| #
dark web market links https://github.com/aresonioncq0a7/aresonion – darkmarket list
Eagle_Voyager
| #
Полное руководство по финансам от А до Я: https://sfera-krasoty.ru
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор качественных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от мелких партий до крупных поставок, мы гарантируем своевременную реализацию вашего заказа.
Каждая единица изделия подтверждена соответствующими документами, подтверждающими их происхождение. Дружелюбная помощь – наша визитная карточка – мы на связи, чтобы улаживать ваши вопросы и адаптировать решения под особенности вашего бизнеса.
Доверьте ваш запрос специалистам РедМетСплав и убедитесь в гибкости нашего предложения
Наша продукция:
Порошок висмутовый C89836 – UNS Порошок висмутовый C89836 – UNS представляет СЃРѕР±РѕР№ высококачественный химический РїСЂРѕРґСѓРєС‚, идеально подходящий для различных промышленных применений. Ртот порошок отличается отличной чистотой Рё стабильностью, что делает его незаменимым РІ металлообработке, фармацевтике Рё электронике. Если РІС‹ ищете надежный Рё эффективный материал, вам стоит купить Порошок висмутовый C89836 – UNS. РћРЅ обладает уникальными свойствами, такими как высокая тепло- Рё электрическая проводимость. РќРµ упустите возможность использовать этот универсальный порошок для СЃРІРѕРёС… проектов. Выберите лучшее качество СЃ Порошком висмутовым C89836 – UNS.
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Партнерство с нашей компанией гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
С широким ассортиментом продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым текущие трудности превращаются в завтрашние возможности.
Наши товары:
Мишень для распыления молибдена
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с высоким уровнем сервиса.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете достоверность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – идеальный вариант для вас.
Наша продукция:
Латунная квадратная профильная труба 55С…55С…12.5 РјРј ЛС 59-1 РўРЈ 48-21-428-84 Приобретите латунные квадратные трубы РѕС‚ производителя РЅР° Редметсплав.СЂС„. РЁРёСЂРѕРєРёР№ выбор, высокое качество, устойчивость Рє РєРѕСЂСЂРѕР·РёРё Рё простота монтажа – РІСЃРµ это делает наши трубы незаменимым решением для ваших проектов.
Wolf_Hacker
| #
Практическое https://kassacredit.ru применение финансов.
WilliamIRrutty
| #
darknet market dark web markets
Donaldlaf
| #
darkmarkets https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark market list
HaroldVew
| #
Читать далее mega мориарти
Kxyufaita
| #
darknet market dark market list
Учетная запись в binance
| #
Your article helped me a lot, is there any more related content? Thanks! https://accounts.binance.com/register?ref=P9L9FQKY
LecenesAppab
| #
Ищете ник инстаграм? Посетите сайт https://sovads.ru/ где вы найдете в широком ассортименте ники инстаграм. Мы занимаемся продажей привлекательных никнеймов и у вас будет красивый ник инстаграм или короткий ник инстаграм для удобства. Стоимость самая лучшая на рынке, а ассортимент магазина пополняется уникальными именами пользователей: словарными, короткими, красивыми и городскими никами. Короткие ники для инстаграм – преимущества очевидно!
Dragon547
| #
Обнаружил, что по финансам лучше всего читать здесь: https://hurma-credit.ru
Toliknaf
| #
darknet markets onion https://github.com/abacuslink4jjku/abacuslink – dark web drug marketplace
MarkNOlaf
| #
dark web markets dark web link
ThunderV9
| #
ТОП-10 ресурсов по финансам: https://lom-ard.ru
GhostByte
| #
Где смотреть актуальные данные о финансах? https://kikosrest.ru
Rabyitabe
| #
darknet markets url https://github.com/darkwebsitesyhshv/darkwebsites – dark web sites
Pingrutty
| #
best darknet markets https://github.com/aresonioncq0a7/aresonion – onion dark website
StormHunter
| #
Приветствую. Подскажите, где посмотреть https://bassein-elektrometallurg.ru информацию о финансах?
Volodyanaf
| #
darknet websites dark market
DonDonfaita
| #
dark web markets https://github.com/nexusdarkrtv1u/nexusdark – dark web market links
WilliamIRrutty
| #
darknet markets links dark web market urls
Cyber357
| #
Хочу понять в финансах. Что https://salgariint.ru посоветуете?
Kxyufaita
| #
dark markets 2025 darknet markets 2025
Wiltonsauff
| #
https://thechampions.ru/
Samuelhox
| #
На официальном сайте Kent Casino предусмотрены самые популярные способы пополнения и вывода средств. Вы сможете использовать банковские карты, электронные кошельки и криптовалюту для удобных и быстрых финансовых операций https://kent777.casino/
https://t.me/Gizbo_zerkalo_ru
| #
Онлайн казино: обзор самых популярных игр с бонусами
Современные платформы азартных развлечений предлагают игрокам множество увлекательных соревнований, каждый из которых имеет свои особенности и заманчивые предложения. Выбор для пользователя становится настоящим испытанием, особенно в условиях разнообразия форматов и интерфейсов. Более того, умение эффективно извлекать выгоду из акций и поощрений – важный аспект успешной игры. Обратите внимание на то, какие привилегии доступны для новичков и постоянных клиентов, чтобы сделать свои ставки максимально выгодными.
Среди трех категорий, к которым стоит обратиться, можно выделить слоты, карточные баталии и живые сессии. Слоты – это не только радость от вращения барабанов, но и широкий выбор тематик, разнообразие игровых функций и щедрые акционные предложения. Карточные конкурсы часто требуют более глубоких стратегий, но они тоже радуют разнообразием форматов и коэффициентов. Живые сессии приносят атмосферу реального заведения прямо к вашему экрану, позволяя взаимодействовать с профессиональными крупье и другими участниками.
Не забывайте исследовать доступные программы лояльности, которые могут значительно увеличить ваши шансы на выигрыш. За каждую ставку вы будете зарабатывать очки, которые затем можно обменивать на реальные деньги или другие привилегии. Это не только повышает интерес к процессу, но и создает дополнительные возможности для призов и подарков. В конечном итоге, грамотный подход к выбору развлечений и использования акций поможет вам получить удовольствие от хобби, а также ощутить финансовые выгоды.
Слоты с щедрыми бонусами
В мире виртуальных развлечений одними из самых привлекательных становятся игровые автоматы, предлагающие богатый набор привилегий. Каждый слот имеет свои уникальные особенности, которые делают его особенно интересным для пользователей.
1. Виды привилегий: Большинство автоматов предоставляют фриспины, что позволит игрокам вращать барабаны без риска. Размер таких вращений варьируется от нескольких до сотен, в зависимости от условий. Опция накопительных бонусов также встречается часто – игроки могут накапливать выигрышные позиции, что делает игру более стратегической.
2. Тематики и их влияние: Слоты с захватывающей тематикой, такие как приключения или мифология, зачастую предлагают повышенные привилегии. К примеру, игры на основе известных фильмов могут включать в себя дополнительные функции, которые активируются при достижении определенных результатов.
3. Интерактивные функции: Современные автоматы часто внедряют мультифункциональные игровые механики. Такие механизмы могут включать мини-игры, где игроки могут увеличивать свои шансы на выигрыш или получать дополнительные призы при выполнении специальных условий.
4. Рекомендуемые игры: Проверьте слоты с уникальными функциями, такими как Big Bad Wolf или Gonzo’s Quest. Эти автоматы отличаются не только визуальным оформлением, но и механикой, что позволяет максимально задействовать предлагаемые преимущества.
5. Стратегия выбора: При выборе игрового автомата стоит обращать внимание на уровень возврата игроку (RTP). Чем выше этот показатель, тем больше вероятность, что машина будет щедрой на выплаты. Выбирайте автоматы с RTP не ниже 96% для увеличения шансов на успех.
Виртуальные автоматы с интересными привилегиями предоставляют игрокам возможность сочетать азарт и стратегию, что делает процесс ещё более увлекательным и физиологически благоприятным. Не забывайте о правилах игры и устанавливайте лимиты, чтобы избежать неприятных ситуаций. Выбор правильного слота может обернуться не только приятным времяпрепровождением, но и солидным выигрышем.
Карточные игры и предложения
Карточные развлекательные мероприятия приобрели большую популярность среди любителей азарта благодаря привлекательному сочетанию стратегии и удачи. Среди них выделяются такие классические варианты, как блэкджек, покер и баккара. Каждая дисциплина предлагает уникальные возможности для игроков, а также заманчивые акционные предложения, способные увеличить банкролл.
Блэкджек – это игра, где удача и тактика идут рука об руку. Многие платформы предлагают специальные действия, такие как «бонус на стол», который дает возможность получить дополнительный кредит при первой ставке. Игроки могут использовать данный вариант, чтобы протестировать различные стратегии, особенно в рамках первых партий.
Покер, в частности, техасский холдем, является особенным направлением, которое требует от участников не только способности к расчету риска, но и навыков блефа. Платформы часто организуют турниры с призовыми фондами, которые значительно превышают обычные ставки. Специальные предложения на участие в таких событиях могут включать бесплатные входы или увеличенные раздачи фишек.
Баккара представляется интересным выбором для тех, кто предпочтительно изучает простые правила. Некоторые онлайн-сервисы предоставляют акции, позволяющие получить возврат части проигранных средств, что делает риски менее ощутимыми. Это создает возможность больше сосредоточиться на процессе игры.
Обратите внимание, что многие учреждения предлагают щедрые стартовые пакеты, которые могут включать не только денежные предложения, но и дополнительные карты для участия в карточных мероприятиях. Важно внимательно изучать условия, так как иногда могут быть ограничения по времени или минимальным ставкам.
Выбор подбираемой стратегии и понимание как работают акционные предложения могут существенно повлиять на общее впечатление от карточных сцен. Разумное использование акций и внимательное отношение к правилам помогут превратить обычный игровой процесс в успешное приключение.
https://t.me/otzyvy7k_bot
MarkNOlaf
| #
darknet markets 2025 dark web market list
Donaldlaf
| #
darknet links https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet site
VortexHunter
| #
История внедрения финансов в проекте: https://crowd-com.ru
GhostNomad
| #
Доступно объяснено о финансах: https://vsekredity-chel.ru
Toliknaf
| #
dark market url https://github.com/abacuslink4jjku/abacuslink – dark websites
RobertDaw
| #
Best non Gamstop casino I’ve played at had fast support—nice!
non gamstop uk casinos with paypal
Sentinel7159
| #
Как https://lpandb.ru войти в тему финансов?
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Закупка у нас гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
С широким ассортиментом продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым сегодняшние вызовы превращаются в завтрашние успехи.
Наши товары:
Керамическая мишень АZO для распыления
Pingrutty
| #
onion dark website https://github.com/aresonioncq0a7/aresonion – dark markets 2025
WilliamIRrutty
| #
darkmarket link darknet site
Rabyitabe
| #
darknet marketplace https://github.com/darkwebsitesyhshv/darkwebsites – dark markets
Wiltonsauff
| #
champion slots официальный сайт
avtolombardekbsig
| #
кредит под птс с плохой кредитной историей
http://euhope.com/employer/epsontario2146/
кредит под залог птс грузового
Pioneer8500
| #
С чего https://alpha-sber.ru начать финансов?
Kxyufaita
| #
dark markets 2025 dark web market urls
BlueSpark
| #
Практическое применение финансов: https://vashi-okna42.ru
DonDonfaita
| #
darkmarket url https://github.com/nexusdarkrtv1u/nexusdark – darkmarket 2025
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог качественных изделий из редких материалов. Не важно, какие объемы вам необходимы – от мелких партий до крупных поставок, мы обеспечиваем своевременную реализацию вашего заказа.
Каждая единица товара подтверждена всеми необходимыми документами, подтверждающими их качество. Превосходное обслуживание – то, чем мы гордимся – мы на связи, чтобы ответить на ваши вопросы а также предоставлять решения под требования вашего бизнеса.
Доверьте ваш запрос профессионалам РедМетСплав и убедитесь в широком спектре предлагаемых возможностей
поставляемая продукция:
Лента танталовая Ta-W10 Лента танталовая Ta-W10 – это высококачественный металл, обладающий исключительными свойствами. РћРЅ устойчив Рє РєРѕСЂСЂРѕР·РёРё Рё идеален для применения РІ условиях высокой температуры Рё агрессивной среды. Рспользуется РІ химической промышленности, Р° также РІ производстве электроники.Лента отлично РїРѕРґС…РѕРґРёС‚ для создания кабелей, прокладок Рё РґСЂСѓРіРёС… деталей, требующих надежности. Доступна РІ различных размерах Рё толщине, что позволяет легко подобрать необходимый вариант для вашего проекта. РќРµ упустите возможность купить Лента танталовая Ta-W10 Рё улучшить качество ваших изделий!
FNDaviditabe
| #
darknet drugs dark markets
1win_rlEr
| #
1 vin https://1win6016.ru .
Warden5625
| #
Как реализовать финансы? https://srg-collection.ru.
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем внушительный выбор продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с непревзойденным обслуживанием.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в выборе товаров и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете достоверность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – ваш лучший выбор.
Наши товары:
Бронзовое внутреннее стопорное пружинное кольцо 62х1.7 мм БрАЖНМц9-4-4-1 ГОСТ 13943-86 Приобретите качественные бронзовые внутренние стопорные кольца по ГОСТ в Редметсплав.рф. Наши колечки отличаются превосходными техническими характеристиками, высоким качеством и надёжностью. Широкий ассортимент и гарантированное соответствие ГОСТ позволят удовлетворить потребности любого заказчика.
bankrotstvo v moskve_yoKi
| #
Узнайте мнение тех, кто уже прошел процедуру банкротства и списал свои долги http://bankrotstvo-v-moskve123.ru .
Andrewopero
| #
бюстгальтер Caitline с сетчатой вставкой Ищете идеальный бюстгальтер? Обратите внимание на Caitline! Отзывы покупательниц подтверждают: это сочетание комфорта и элегантности. Широкий размерный ряд, включая модели для больших размеров, позволяет каждой женщине найти подходящий вариант. В интернет-магазине Caitline вас ждет разнообразие моделей: с эффектом пуш-ап, для повседневной носки, с кружевом, для спорта и даже для особых случаев, таких как свадьба или вечерний выход.
binance registration
| #
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
1win_kbEr
| #
1win официальный сайт регистрация 1win официальный сайт регистрация .
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
Обладая обширной линейкой продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Приступая к взаимодействию с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым сегодняшние вызовы превращаются в завтрашние успехи.
Наши товары:
Гранулы ниобия 4N
Donaldlaf
| #
dark market https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet market list
Overlord7009
| #
Ошибки новичков в финансах: https://vista-profi.ru
Volodyanaf
| #
dark market 2025 darknet marketplace
Timothywax
| #
Основной вопрос импортеров в России сводится к одному: как оплатить инвойс за рубеж быстро и безопасно? Опасаясь штрафов и вторичных санкций, зарубежные банки пристально проверяют платежи из РФ, чтобы Ваши платежи в срок доходили до контрагента, и обходили блокировки и задержки, на сегодня существует рабочий способ – оплата через платежного агента. Кто такой платежный агент и как с ним работать мы рассказали на наших площадках Оплата инвойсов в Азии
Pingrutty
| #
darkmarket 2025 https://github.com/aresonioncq0a7/aresonion – dark web markets
WolfX6
| #
Как не сдаться при изучении финансов: https://24rd.ru
Toliknaf
| #
darkmarket https://github.com/abacuslink4jjku/abacuslink – dark web market links
WilliamIRrutty
| #
darknet market list dark market link
Omega_Spark
| #
Типичные промахи в https://aktivedengi.ru финансах.
1win_qdEr
| #
1wi. http://1win6016.ru .
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Имея широкий спектр продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие всем нормам.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит надежного партнера, с которым текущие трудности превращаются в завтрашние успехи.
Наши товары:
Гранулы высокочистого ванадия
Kxyufaita
| #
darkmarket 2025 darkmarket list
mostbet_ghSn
| #
мостбет казино войти мостбет казино войти .
DavidJelry
| #
Przejrzalem portal Nelja i rozczarowalem sie. wyglad jest archaiczny, obsluga jest trudne, a przydatnych tresci jest malo. ciagle awarie, wszystko sie zacina, natretny spam przeszkadzaja w uzywanie. Bez watpliwosci, ze witryna jest martwa, poniewaz nic nie jest poprawiane. Brak pomocy, linki sa uszkodzone. Jesli potrzebujesz wartosciowego serwisu, lepiej jej unikac!
Rabyitabe
| #
onion dark website https://github.com/darkwebsitesyhshv/darkwebsites – darknet drugs
Sentinel1710
| #
Авторитетное мнение о финансах: https://mir-aquariuma72.ru
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор качественных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до обширных поставок, мы обеспечиваем своевременную реализацию вашего заказа.
Каждая единица продукции подтверждена требуемыми документами, подтверждающими их качество. Опытная поддержка – наша визитная карточка – мы на связи, чтобы улаживать ваши вопросы по мере того как находить ответы под особенности вашего бизнеса.
Доверьте ваш запрос профессионалам РедМетСплав и убедитесь в множестве наших преимуществ
Наша продукция:
РљСЂСѓРі ниобиевый 50БТЦ-Р’Р” РљСЂСѓРі ниобиевый 50БТЦ-Р’Р” – это высококачественный РїСЂРѕРґСѓРєС‚, разработанный для осуществления операций СЃ ниобием. Ртот РєСЂСѓРі обладает уникальными свойствами, которые обеспечивают высокую прочность Рё РєРѕСЂСЂРѕР·РёРѕРЅРЅСѓСЋ стойкость. Благодаря СЃРІРѕРёРј характеристикам, РљСЂСѓРі ниобиевый 50БТЦ-Р’Р” идеально РїРѕРґС…РѕРґРёС‚ для использования РІ различных промышленных сферах, включая авиастроение Рё атомную энергетику. Р’С‹ можете купить РљСЂСѓРі ниобиевый 50БТЦ-Р’Р”, чтобы обеспечить надежность Рё долговечность СЃРІРѕРёС… изделий. РќРµ упустите возможность улучшить качество своей продукции!
OmegaV3
| #
Шутки про финансы: https://rezinplit.ru
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Закупка у нас гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
Имея широкий спектр редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым текущие трудности превращаются в завтрашние возможности.
Наша продукция:
Гранулы ниобия 4N
FNDaviditabe
| #
tor drug market dark web link
MarkNOlaf
| #
darknet markets 2025 dark markets
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы обеспечить любой запрос с непревзойденным обслуживанием.
Наша команда поддержки всегда на связи, чтобы помочь вам в подборе нужных изделий и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете достоверность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – ваш лучший выбор.
поставляемая продукция:
Лист судостроительный 24x1000x3000 D EN10025:2 Приобретите высококачественные листы судостроительные от компании Редметсплав.рф для надежного и долговечного использования в вашем судостроительном проекте. Различные размеры и толщины, прочность и устойчивость к коррозии делают эти листы идеальным выбором для любого судостроительного проекта.
mostbet_luSn
| #
мрстбет https://mostbet789.ru/ .
RobertDaw
| #
Casino non Gamstop platforms are perfect for casual gaming—love it!
casino non on gamstop
mostbet_ngen
| #
mostber ashapiter0.forum24.ru/?1-19-0-00001444-000-0-0-1742819001 .
DonDonfaita
| #
darknet drug market https://github.com/nexusdarkrtv1u/nexusdark – dark websites
1win_msKi
| #
1вин онлайн https://zdorovie.forum24.ru/?1-7-0-00000231-000-0-0-1742818050 .
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Партнерство с нашей компанией гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Обладая обширной линейкой продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым сегодняшние вызовы превращаются в завтрашние достижения.
Наши товары:
Керамическая мишень для распыления Р±РѕСЂР° – плоская
GeorgeTom
| #
Source:
– royaljackpotcasino.org
porno_hyKt
| #
порно порно .
DewurKiz
| #
Компания Глобал ЖБИ https://global-gbi.ru/ является крупным производителем изделий из бетона и железобетона в Санкт-Петербурге. Посетите наш сайт, ознакомьтесь с широкой номенклатурой производимого товара по выгодным ценам. Мы осуществляем оперативную и быструю доставку продукции. Также на заводе ЖБИ, можно заказать производство конструкций разного типа и назначения для строительства дорог, взлетных полос, тротуаров, пешеходных зон, зданий, сооружений, внутридомовых конструкций, инженерных коммуникаций и т.д.
Cyber_Nomad
| #
Факты и вымысел о https://kpk-kk.ru финансах.
MatthewHen
| #
Онлайн-казино 7k — это продвинутая платформа для игр с ставками с широким выбором игр. Здесь можно найти известные видеослоты, настольные игры и игры с живыми дилерами с опытными дилерами. Дизайн пространный и простой в навигации, что делает игру приятной и увлекательной. На https://7k740.casino/ru/ предусмотрены классные призы для любителей азартных игр. В целом, казино предоставляет отличный опыт для каждого игрока.
mostbet_ooen
| #
мостбет скачать на андроид https://ashapiter0.forum24.ru/?1-19-0-00001444-000-0-0-1742819001 .
Alpha_Guardian
| #
Нестандартный подход к финансам: https://perspectiva-leasing.ru
1win_htKi
| #
1win прямой эфир zdorovie.forum24.ru/?1-7-0-00000231-000-0-0-1742818050 .
urwork
| #
Юридические услуги https://urwork.ru в Санкт-Петербурге и Москве – от консультации до защиты интересов в суде. Оперативно, надежно и с гарантией конфиденциальности.
Alpha707
| #
Простой язык о финансах: https://narodnakassa.ru
Pingrutty
| #
onion dark website https://github.com/aresonioncq0a7/aresonion – dark web market
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог качественных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до обширных поставок, мы обеспечиваем своевременную реализацию вашего заказа.
Каждая единица товара подтверждена требуемыми документами, подтверждающими их качество. Превосходное обслуживание – наша визитная карточка – мы на связи, чтобы разрешать ваши вопросы по мере того как предоставлять решения под требования вашего бизнеса.
Доверьте ваш запрос специалистам РедМетСплав и убедитесь в гибкости нашего предложения
поставляемая продукция:
РџРѕРєРѕРІРєР° вольфрамовая РР’Р-3 РџРѕРєРѕРІРєР° вольфрамовая РР’Р-3 представляет СЃРѕР±РѕР№ высококачественный РїСЂРѕРґСѓРєС‚, используемый РІ различных отраслях. РћРЅР° обладает отличными механическими свойствами Рё высокой термостойкостью, что делает ее идеальным выбором для производства деталей, работающих РІ экстремальных условиях. РџРѕРєРѕРІРєР° гарантирует надежность Рё долговечность, благодаря чему становится незаменимой для РјРЅРѕРіРёС… промышленных приложений. Если РІС‹ хотите обеспечить эффективность своей работы, вам стоит купить РџРѕРєРѕРІРєР° вольфрамовая РР’Р-3. Получите РїСЂРѕРґСѓРєС‚, который отвечает высоким стандартам качества Рё надежности.
Zooma казино сайт
| #
Добро пожаловать в Zooma Casino – современная игровая платформа с уникальными возможностями! Здесь вас ждут лучшие азартные развлечения от ведущих разработчиков. https://zooma-play.buzz/ и погрузитесь в мир высоких выигрышей!
Что делает Zooma Casino идеальным выбором?
Популярные слоты с бонусными раундами.
Программы лояльности с кэшбеком и фриспинами.
Гарантированные выигрыши с максимальной безопасностью.
Адаптивная мобильная версия без зависаний и лагов.
Дружелюбные операторы готовы ответить на все вопросы.
Присоединяйтесь к Zooma Casino и воспользоваться всеми преимуществами!
mostbet_suSn
| #
mostbet скачать на телефон бесплатно андроид https://mostbet789.ru/ .
Omega_Spark
| #
Лучшее что https://buhprofihelp.ru находил по теме финансов.
Donaldlaf
| #
dark web marketplaces https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – tor drug market
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с прекрасным качеством обслуживания.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – идеальный вариант для вас.
Наши товары:
Медный тройник РїРѕРґ пайку СЃ направленным отводом 28С…23С…0.9 РјРј 18.4С…20.4 РјРј твердая пайка Рњ2Р Рњ ГОСТ Р 52922-2008 Купить качественные медные тройники РїРѕРґ пайку для надежных Рё долговечных соединений РІ трубопроводных системах. Разнообразие размеров Рё конфигураций, высокая теплопроводность Рё устойчивость Рє РєРѕСЂСЂРѕР·РёРё. Простая установка Рё надежность соединения. Рдеальное решение для различных проектов. Редметсплав.СЂС„
nvblfurn
| #
https://blotos.ru/news/tri_v_ryad__igrayte_onlayn_besplatno_i_bez_registracii.html кто ты из гарри поттера тест
avtolombardkazancoN
| #
автоломбард под залог
https://icmimarlikdergisi.com/kariyer/companies/zaim-pod-zalog-pts-19150/
кредит под залог машины
WilliamIRrutty
| #
dark web marketplaces darknet drug store
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог качественных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от мелких партий до обширных поставок, мы гарантируем своевременную реализацию вашего заказа.
Каждая единица продукции подтверждена соответствующими документами, подтверждающими их происхождение. Дружелюбная помощь – наша визитная карточка – мы на связи, чтобы ответить на ваши вопросы а также адаптировать решения под особенности вашего бизнеса.
Доверьте вашу потребность в редких металлах профессионалам РедМетСплав и убедитесь в множестве наших преимуществ
Наша продукция:
РўСЂСѓР±Р° магниевая ISO-MC21210 – BS ISO 16220 Пруток магниевый ISO-MC21210 – BS ISO 16220 идеально РїРѕРґС…РѕРґРёС‚ для различных промышленных приложений благодаря СЃРІРѕРёРј отличным механическим свойствам Рё легкости. Ртот материал обладает высокой РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ стойкостью Рё отличается хорошей свариваемостью, что делает его популярным выбором РІ обработке металлов. Если РІС‹ ищете надежный Рё качественный РїСЂРѕРґСѓРєС‚, то РїРѕРєСѓРїРєР° прутка магниевого ISO-MC21210 – BS ISO 16220 станет отличным решением для ваших нужд. РќРµ упустите возможность обеспечить СЃРІРѕР№ проект качественным материалом!
mostbet_ynen
| #
mostbet http://www.ashapiter0.forum24.ru/?1-19-0-00001444-000-0-0-1742819001 .
Toliknaf
| #
darknet sites https://github.com/abacuslink4jjku/abacuslink – darknet markets links
1win_hlKi
| #
1win официальный http://zdorovie.forum24.ru/?1-7-0-00000231-000-0-0-1742818050/ .
Sarozhdab
| #
На сайте https://prokat.car-trip.ru/ вы сможете выбрать автомобиль для того, чтобы воспользоваться прокатом. Только сейчас действует скидка 10% на определенные автомобили. Есть те машины, которые относятся к классу Комфорт, Бизнес, Стандарт. В автопарке имеются и автодома, кабриолеты. Высококлассные менеджеры всегда находятся на связи, чтобы вы задали им любой вопрос. Отсутствуют скрытые платежи, нет залога, ограничения относительно пробега. Все клиенты получают право воспользоваться дисконтной картой.
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для оформления товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы обеспечить любой запрос с непревзойденным обслуживанием.
Наша команда поддержки всегда на связи, чтобы помочь вам в выборе товаров и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете достоверность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – идеальный вариант для вас.
Наши товары:
Бронзовое внутреннее стопорное пружинное кольцо 200х3 мм БрБНТ1.9 ГОСТ 13943-86 Приобретите качественные бронзовые внутренние стопорные кольца по ГОСТ в Редметсплав.рф. Наши колечки отличаются превосходными техническими характеристиками, высоким качеством и надёжностью. Широкий ассортимент и гарантированное соответствие ГОСТ позволят удовлетворить потребности любого заказчика.
Kxyufaita
| #
darkmarket list darknet sites
Byte3012
| #
Авторитетное мнение о финансах: https://dolganet-nn.ru
RedReaper
| #
Где брать актуальные данные о финансах? https://zoovetbor.ru
FNDaviditabe
| #
dark market link darknet markets links
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор высококачественных изделий из ценных материалов. Не важно, какие объемы вам необходимы – от мелких партий до масштабных поставок, мы обеспечиваем своевременную реализацию вашего заказа.
Каждая единица изделия подтверждена соответствующими документами, подтверждающими их качество. Превосходное обслуживание – наша визитная карточка – мы на связи, чтобы разрешать ваши вопросы а также предоставлять решения под специфику вашего бизнеса.
Доверьте вашу потребность в редких металлах специалистам РедМетСплав и убедитесь в широком спектре предлагаемых возможностей
Наши товары:
Пруток молибденовый ЦМ-2Рђ Пруток молибденовый ЦМ-2Рђ – это высококачественный металл, обладающий отличной прочностью Рё жаростойкостью. РћРЅ широко используется РІ различных отраслях, включая аэрокосмическую Рё промышленную. Молибденовые прутки применяются для создания деталей, которые подвергаются высокому температурному воздействию. Ртот материал устойчив Рє РєРѕСЂСЂРѕР·РёРё Рё обеспечивает надежность РІ эксплуатации. Если РІС‹ ищете надежное решение для СЃРІРѕРёС… проектов, вам стоит купить Пруток молибденовый ЦМ-2Рђ. Выберите качественный РїСЂРѕРґСѓРєС‚, который удовлетворит РІСЃРµ ваши требования.
MarkNOlaf
| #
dark web marketplaces dark market list
Master3905
| #
Эксклюзивное интервью с экспертом по финансам: https://lombard-gran.ru
Punuyuseks
| #
Доставка комплексных обедов в Алматы для ваших сотрудников или на дом на сайте https://bvd.kz/ – у нас широкий ассортимент и выбор блюд по отличным ценам! Ознакомьтесь на сайте с составами и ценами на комплексные обеды. Заключаем договоры на доставку обедов в офисы и административные учреждения, доставляем обеды в школы и детские сады, где нет собственной кухни, в магазины и прочие подобные учреждения, у сотрудников которых нет возможности пообедать в столовой. Подробнее на сайте.
Rabyitabe
| #
dark market 2025 https://github.com/darkwebsitesyhshv/darkwebsites – darkmarket link
Volodyanaf
| #
darknet markets url darknet market list
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с прекрасным качеством обслуживания.
Наша команда поддержки всегда на связи, чтобы помочь вам в подборе нужных изделий и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – наилучшее решение для вашего бизнеса.
Наша продукция:
Медный стандартный однораструбный отвод 90 градусов под пайку 35х29х1 мм 23х25 мм мягкая пайка М2т ГОСТ Р52922-2008 Покупайте качественные медные однораструбные отводы 90 градусов под пайку от Редметсплав. Надежные и долговечные соединения для водоснабжения, отопления и кондиционирования. Быстрая установка и эстетичный вид. Доставка по России.
Steel208
| #
Может кто знает разбирается в финансах? Куда зайти? https://weddingkms.ru
Vickiuseds
| #
Popular Aviator game is a next-gen online game where results depend on your strategic timing.
How to play: a plane starts rising into the sky, and with every second, the reward grows higher.
Your goal is to press “Stop” before the plane flies away. If you’re fast enough — you collect your prize. Wait too long — and you lose everything.
That’s why Aviator is a balance of skill and luck.
Visit https://9323fb.com/how-to-deposit-money-to-aviator-2024-fast-safe-beginner-friendly/ to learn how to make fast, safe, and beginner-friendly deposits for Aviator.
Play with ease thanks to:
– a user-friendly interface
– no-delay transfers
– anytime support service
– cross-device flexibility
Play whenever you want and win big before the plane disappears!
RandyMup
| #
https://лучшие-адвокаты-москвы.рф
1xbet_noPi
| #
Откройте для себя мир ставок с 1xbet, начать играть.
Добро пожаловать в мир ставок с 1xbet, в индустрии.
1xbet предлагает щедрые бонусы, акции.
Скорее ставьте на свои любимые команды с 1xbet, наслаждайтесь.
1xbet – ваш портал в мир лайв-ставок, вы всегда на шаг впереди.
1xbet предлагает широкую линейку ставок, создавайте.
На 1xbet найдётся ставку для каждого, от спорта до киберспорта.
Смотрите матчи в режиме реального времени с 1xbet, наслаждайтесь просмотром.
Деньги на вашем счете с 1xbet за считанные минуты, не ждите.
Получите инсайдерскую информацию с 1xbet, будьте всегда на шаг впереди.
Ставьте с уверенностью на 1xbet, мы ценим вашу конфиденциальность.
Промокоды и специальные предложения на 1xbet, воспользуйтесь шансом.
Ставьте смело с 1xbet, выберите 1xbet для своей игры.
Чат поддержки 24/7 на 1xbet, мы рядом, чтобы помочь.
Регулярные турниры и конкурсы на 1xbet, воспользуйтесь шансом.
Ставьте в любое время и в любом месте с 1xbet, сделайте ставки на ходу.
Дайте себе преимущества с 1xbet, будьте стратегом.
Простая регистрация на 1xbet, доступ к азарту.
1xbet – это азарт, который ждет вас, начните выигрывать.
Не упустите уникальные возможности на 1xbet, развивайте свои навыки.
Keyword https://1xbet-login-egypt.com/ .
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Работа с нами гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа требуемого количества.
Имея широкий спектр продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит надежного партнера, с которым сегодняшние вызовы превращаются в завтрашние успехи.
Наша продукция:
Мишень для распыления сплава AlCu
DonDonfaita
| #
dark web marketplaces https://github.com/nexusdarkrtv1u/nexusdark – darkmarket list
Samuellem
| #
Запросите персональную консультацию по выбору экспонатов – узнайте больше информации https://blacksocially.com/Artefactad
Pingrutty
| #
dark markets https://github.com/aresonioncq0a7/aresonion – darknet markets onion address
Guardian9721
| #
Интерактивный курс по https://vnagaevo.ru финансам.
StormStriker
| #
Подборка ресурсов по финансам: https://samara-investor.ru
WilliamIRrutty
| #
darknet drug store darknet websites
Cyber332
| #
Как выбрать лучшее решение https://kpkazbuka.ru для финансов.
Donaldlaf
| #
darkmarket list https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet market lists
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Обладая обширной линейкой продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Приступая к взаимодействию с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым сегодняшние вызовы превращаются в завтрашние достижения.
Наша продукция:
Алюминиево-хромовая мишень для распыления (AlCr)
OmegaStriker
| #
Что нельзя делать с https://rtsfi.ru финансами?
Deyiramnaify
| #
Ищете фильтры для воды, септики, смесители, насосы? Посетите https://shoph2o.ru/ где вы сможете найти и купить в интернет магазине «H2O – Территория чистой воды» – системы водоочистки любой сложности. У нас широкий ассортимент продукции по выгодным ценам и быстрой доставкой. Также у нас можно заказать услуги анализа воды, монтаж септиков, ремонт систем водоподготовки и фильтров для воды. И их сервисное обслуживание.
Kxyufaita
| #
dark market onion dark web market list
Toliknaf
| #
darknet markets url https://github.com/abacuslink4jjku/abacuslink – dark web market urls
MarkNOlaf
| #
bitcoin dark web darknet drugs
FNDaviditabe
| #
dark market list darkmarket 2025
Steel680
| #
Обнаружил, что по финансам лучше https://realtor38.ru всего смотреть здесь.
vetivsiz
| #
Автосервис в СПб СТО Приморский большой спектр услуг предлагает. Мы поможем починить неисправность в автомобиле. Гарантируем доступные цены и высочайшее качество работы. При необходимости профессионально проконсультируем вас. Ищете автосервис питер? Sto812.com – тут полезная информация представлена. На сайте вы увидите ряд важных проблем, которые появляются при приобретении некачественных фильтрующих элементов. Узнаете, какими свойствами обладает тормозная жидкость. Обладаем необходимым опытом и инструментом. Работаем быстро. Обращайтесь к нам!
CharlesZoony
| #
Информационный портал посвященный обучению стройке и ремонту своими руками. Лучшие материалы на тему большого строительства, а также интересные советы по дизайну https://aquabiodesign.ru/
Rabyitabe
| #
darknet markets onion https://github.com/darkwebsitesyhshv/darkwebsites – dark web markets
Reaper1715
| #
Забавные моменты в финансах: https://amalfd.ru
BohacMig
| #
На сайте https://face-me.ru/ вы сможете попробовать все возможности нейросетей и всего за несколько секунд превратить любое фото в видео. Инновационные и уникальные технологии дают возможность получить роскошную, яркую и любопытную фотосессию без профессионального оборудования, студии. Вы сможете создать фантастические образы и удивительные портреты в соответствии с предпочтениями. Эта программа дарит вам огромное количество возможностей и вдохновение. Вы каждый раз будете получать удивительные, красивые образы, которые всегда будут с вами.
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор отборных изделий из ценных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до масштабных поставок, мы гарантируем быстрое выполнение вашего заказа.
Каждая единица изделия подтверждена всеми необходимыми документами, подтверждающими их соответствие стандартам. Опытная поддержка – наша визитная карточка – мы на связи, чтобы улаживать ваши вопросы и находить ответы под особенности вашего бизнеса.
Доверьте потребности вашего бизнеса специалистам РедМетСплав и убедитесь в широком спектре предлагаемых возможностей
Наши товары:
РўСЂСѓР±Р° магниевая MgTh3Zn2Zr Пруток магниевый MgTh3Zn2Zr представляет СЃРѕР±РѕР№ уникальный легированный сплав, который отличается высокой прочностью Рё РЅРёР·РєРёРј весом. Рспользуется РІ aerospace, автомобильной Рё энергетической отраслях. Данный материал обладает отличной РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ стойкостью Рё хорошей сваркой, что делает его идеальным выбором для создания прочных конструкций. Если РІС‹ хотите улучшить эффективность СЃРІРѕРёС… проектов, то вам нужно купить Пруток магниевый MgTh3Zn2Zr. Ртот РїСЂРѕРґСѓРєС‚ обеспечит надежность Рё долговечность, необходимые для современных решений РІ инженерии.
1win_rnPr
| #
1 win вход https://1win6017.ru .
Pingrutty
| #
bitcoin dark web https://github.com/aresonioncq0a7/aresonion – dark web markets
ZecohSwexy
| #
ТТК — транспортная компания, специализирующаяся на перевозках автомобилей на автовозах по всей России. Наша компания имеет опыт перевозок автовозами более 12 лет. Свыше 40 единиц техники позволяют осуществлять регулярные рейсы по всей России. У нас можно заказать автовозы по России для транспортировки автомобиля любого типа: седана, универсала, кроссовера, внедорожника и даже автомобиля в аварийном состоянии. Качественный сервис по доступным ценам. Обеспечиваем высокое качество и безопасность на каждом этапе перевозки. Широкая география перевозок позволяет обеспечить перевозку машин в любую точку страны: Москва, Санкт-Петербург, Владивосток, Екатеринбург, Иркутск, Казань, Кемерово, Краснодар, Красноярск, Новосибирск, Омск, Пермь, Ростов-на-Дону, Самара, Симферополь, Сочи, Ставрополь, Сургут, Тольятти, Томск, Тюмень, Улан-Удэ, Уфа, Хабаровск, Челябинск, Чита и в другие города России. Практически во всех городах России мы имеем специальные охраняемые стоянки для авто. Работаем 24/7. Сайт нашей компании ТТК: https://avtovoz7.ru/
NightZ2
| #
https://success-finance-ipoteka.ru Какие платформы использовать для финансов?
Dragon_Master
| #
Мемы про финансы: https://kpk-cfp.ru
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф гарантирует все стадии сделки, предоставляя полный пакет необходимых документов для оформления товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с непревзойденным обслуживанием.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в выборе товаров и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете достоверность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – наилучшее решение для вашего бизнеса.
Наша продукция:
Двухраструбный медный обвод под пайку 80х68х1.8 мм 35.5х38.5 мм твердая пайка М2т ГОСТ Р52922-2008 Выберите качественные Медные двухраструбные обводы под пайку различных размеров и диаметров. Надежное соединение труб, высокая устойчивость к коррозии и превосходная теплопроводность. Узнайте больше о нашей продукции и найдите оптимальное решение для вашего проекта.
DonDonfaita
| #
dark market url https://github.com/nexusdarkrtv1u/nexusdark – darknet markets links
avtolombardkazancoN
| #
займ под залог машины
https://emploi-securite.com/societes/jobsandbussiness4839/
займ под птс казань
Derricksaisy
| #
Флорист создал настоящий цветочный шедевр
доставка цветов в томске
1win_gpPr
| #
1вин кыргызстан http://www.1win6017.ru .
Volodyanaf
| #
dark web market links darknet markets
RobertDaw
| #
Non Gamstop casinos no deposit offers are my new favorite thing—sweet!
non-gamstop casino no deposit codes
WilliamIRrutty
| #
dark web market darkmarket
Red_Reaper
| #
Подробный разбор финансов: https://zaimsosed.ru
DragonV7
| #
Как реализовали финансов https://kpkbelkred.ru в компании.
Donaldlaf
| #
dark markets 2025 https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet market list
Kxyufaita
| #
dark web drug marketplace dark web market urls
viaplay
| #
certainly like your web site however you need to test the spelling
on quite a few of your posts. Several of them
are rife with spelling problems and I in finding it very bothersome to tell the
reality then again I will certainly come again again.
My page – viaplay
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог отборных изделий из ценных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до масштабных поставок, мы гарантируем быстрое выполнение вашего заказа.
Каждая единица продукции подтверждена соответствующими документами, подтверждающими их происхождение. Превосходное обслуживание – то, чем мы гордимся – мы на связи, чтобы ответить на ваши вопросы а также находить ответы под специфику вашего бизнеса.
Доверьте вашу потребность в редких металлах специалистам РедМетСплав и убедитесь в множестве наших преимуществ
поставляемая продукция:
РўСЂСѓР±Р° магниевая G-A9Z1Y4 – AFNOR NF A57-705 Пруток магниевый G-A9Z1Y4 – AFNOR NF A57-705 представляет СЃРѕР±РѕР№ высококачественный РїСЂРѕРґСѓРєС‚, обладающий отличными механическими свойствами Рё стойкостью Рє РєРѕСЂСЂРѕР·РёРё. РћРЅ идеально РїРѕРґС…РѕРґРёС‚ для использования РІ различных отраслях, включая авиастроение, автомобилестроение Рё РґСЂСѓРіРёРµ высокотехнологичные сферы. Благодаря своей легкости, пруток магниевый G-A9Z1Y4 – AFNOR NF A57-705 способствует снижению веса конструкции, что делает его особенно востребованным. РќРµ упустите возможность купить Пруток магниевый G-A9Z1Y4 – AFNOR NF A57-705 для повышения эффективности ваших проектов.
FNDaviditabe
| #
dark market 2025 best darknet markets
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Партнерство с нашей компанией гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа требуемого количества.
Имея широкий спектр редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие всем нормам.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Приступая к взаимодействию с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым текущие трудности превращаются в завтрашние успехи.
Наши товары:
Керамическая мишень Mos2, плоская
Master7176
| #
Малоизвестные фишки финансов: https://evs-ufa.ru
Toliknaf
| #
darknet markets https://github.com/abacuslink4jjku/abacuslink – darkmarket list
GhostReaper
| #
Лично мне помог этот ресурс по финансам: https://grozfinline.ru
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем внушительный выбор продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы поставить любой запрос с непревзойденным обслуживанием.
Наша команда поддержки всегда на связи, чтобы помочь вам в выборе товаров и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – ваш лучший выбор.
Наша продукция:
Титановая труба 105х5.5х1500 мм ПТ1М ГОСТ 22897-86 Откройте мир прочных и надежных титановых труб с компанией Редметсплав. Наши высококачественные титановые трубы сочетают в себе устойчивость к коррозии, износостойкость и легкий вес, делая их незаменимым материалом для применения в различных сферах промышленности. Широкий выбор различных диаметров и толщин, а также индивидуальные решения для вашего производства. Гарантированное качество и надежность поставок.
1win_lmPr
| #
1win. pro http://1win6017.ru/ .
Pioneer4738
| #
5 главных ошибок при работе https://stafpro.ru с финансами.
Pingrutty
| #
dark market link https://github.com/aresonioncq0a7/aresonion – darknet market
ShaneJerve
| #
https://firelak.ru В современном строительстве и отделке, где дерево занимает важное место, обеспечение пожарной безопасности становится приоритетной задачей. Огнезащитный лак – это эффективное решение для защиты деревянных конструкций и элементов интерьера от возгорания и распространения пламени.
Rabyitabe
| #
darknet markets 2025 https://github.com/darkwebsitesyhshv/darkwebsites – darknet markets onion
Blue_Master
| #
Практическое https://dolce-vita74.ru применение финансов.
BillyBor
| #
Забудьте о дискомфорте и неуверенности – имплантация зубов в нашей клинике возвращает естественную красоту и функциональность вашей улыбки http://forum.omnicomm.pro/index.php?action=profile;u=16464
Samuellem
| #
Экспонаты с подтвержденной подлинностью и экспертной оценкой – дополнительные сведения тут http://www.askmap.net/location/7303484/%D0%BE%D0%B0%D1%8D/artefactum-gallery
BillyBor
| #
Наши специалисты подбирают индивидуальные решения для каждого пациента, предлагая лучшие методики имплантации зубов и протезирования https://a6-allroad.ru/member.php?u=14441
WilliamIRrutty
| #
darknet market lists dark market
DonDonfaita
| #
dark websites https://github.com/nexusdarkrtv1u/nexusdark – darkmarket
Hacker2295
| #
Обнаружил, что https://partnerinvest40.ru по финансам лучше всего смотреть здесь.
SteelVoyager
| #
Современные методы по финансам: https://deshevosantehnika.ru
Red284
| #
https://leasing-sibir.ru Новые подходы в финансах.
HarryTaips
| #
Лучший генератор QR-кодов
Kxyufaita
| #
darkmarket list darknet markets
MarkNOlaf
| #
darkmarket url dark market url
JakohlSed
| #
На сайте https://fguard.ru/ почитайте статьи на самую разную тему. К примеру, кибермошенничество, для каких целей используется сеточка, которая на двери микроволновки. Здесь представлена вся нужная информация о смарт-часах и о том, чем они отличаются от бизнес-браслетов. Для того чтобы подыскать нужную информацию, воспользуйтесь специальным рубрикатором. На сайте вы найдете записи, которые касаются нейросетей, гаджетов. Представлены и интересные рекомендации, которые будут необходимы каждому. Имеются данные и про технологии.
FNDaviditabe
| #
darknet marketplace dark market list
Donaldlaf
| #
darknet markets 2025 https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet markets 2025
Volodyanaf
| #
dark web drug marketplace darkmarket link
Pingrutty
| #
dark market 2025 https://github.com/aresonioncq0a7/aresonion – darknet marketplace
DaltonJasse
| #
seo оптимизация цена https://prodvizhenietargeting.ru
EdwardCen
| #
поисковое продвижение сайта заказать https://prodvizheniestatya.ru
Toliknaf
| #
dark market https://github.com/abacuslink4jjku/abacuslink – darkmarket link
SteveViche
| #
сео оптимизация сайта купить seoveb-marketing.ru
JustinCoict
| #
Как получить китайскую визу Планируете поездку в Китай? Наш визовый центр предоставляет полный спектр услуг по оформлению виз в Китай для различных целей: туризм, бизнес, учеба. Мы оказываем профессиональные консультации по всем вопросам, связанным с визовым режимом Китая и подготовкой необходимых документов. Мы поможем вам оформить туристическую визу, бизнес-визу, а также визу для студентов. Наши специалисты подробно проконсультируют вас о необходимых документах, сроках получения визы и ответят на все ваши вопросы. Мы также предлагаем услуги экспресс-визы для тех, кому требуется срочное оформление.
Eagle_Master
| #
Забавные моменты https://gretanv.ru в финансах.
Xirodrdaf
| #
Новини та інформація про місто Кропивницький. Сучасний портал https://kropyvnytskyi.kr.ua/ про місто Кропивницький. Актуальна довідкова інформація про місто, новини, публікації.
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог отборных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до обширных поставок, мы обеспечиваем быстрое выполнение вашего заказа.
Каждая единица продукции подтверждена требуемыми документами, подтверждающими их происхождение. Опытная поддержка – наш стандарт – мы на связи, чтобы улаживать ваши вопросы и находить ответы под требования вашего бизнеса.
Доверьте вашу потребность в редких металлах специалистам РедМетСплав и убедитесь в гибкости нашего предложения
поставляемая продукция:
Полоса магниевая MAG 121 – BS 3370 Порошок магниевый MAG 12 – BS 2970 – это высококачественный РїСЂРѕРґСѓРєС‚, предназначенный для различных промышленных Рё научных задач. РћРЅ обладает отличными антикоррозийными свойствами Рё значительно улучшает характеристики конечных изделий. Р’ производстве используемый магний отличается стабильностью Рё чистотой, что обеспечивает высокую эффективность. Рщете надежный Рё качественный порошок? Купить Порошок магниевый MAG 12 – BS 2970 стоит, чтобы оценить РІСЃРµ его преимущества РІ эксплуатации Рё долговечности. Рто идеальный выбор для тех, кто ценит качество Рё надежность РІ СЃРІРѕРёС… проектах.
RobertDaw
| #
Non Gamstop casino no deposit bonus got me started—love it!
uk casino no gamstop
ShadowProtocol
| #
Как монетизировать финансы https://izumrud585.ru в бизнесе?
Rabyitabe
| #
darknet markets 2025 https://github.com/darkwebsitesyhshv/darkwebsites – dark web market list
HarryTaips
| #
бесплатный онлайн генератор QR кодов
WilliamIRrutty
| #
dark market 2025 dark market onion
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы обеспечить любой запрос с непревзойденным обслуживанием.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в подборе нужных изделий и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – ваш лучший выбор.
Наша продукция:
Контакт РёР· драгоценных металлов платиновый 2С…9 РјРј ПлРд90-10 РўРЈ Рзысканные контакты РёР· золота, серебра Рё платины РѕС‚ Редметсплав. Высочайшее качество, уникальный дизайн Рё эксклюзивные предложения для постоянных клиентов. Добавьте роскошь Рё стиль вашему образу!
HarryTaips
| #
https://qrkoder.ru/
DonDonfaita
| #
onion dark website https://github.com/nexusdarkrtv1u/nexusdark – darknet links
Kxyufaita
| #
darkmarket list bitcoin dark web
Felixvah
| #
https://logistt.ru/services/delivery/china
FNDaviditabe
| #
darkmarket link darkmarket
Omega_Nomad
| #
Памятка для работы с финансами: https://avtokompleks222.ru
avtolombardkemerovosig
| #
срочный кредит под залог птс
https://mastercare.care/employer/avtolombard-zalog-invest9408/
автоломбард залог птс
Pingrutty
| #
dark web drug marketplace https://github.com/aresonioncq0a7/aresonion – dark web markets
Donaldlaf
| #
darknet links https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark web marketplaces
IronZ7
| #
Полное руководство https://delovoipodhodzaim.ru по финансам от А до Я.
Volodyanaf
| #
dark web drug marketplace darkmarket list
MichaelFus
| #
пегас туроператор Компания пегас является одним из лидеров рынка и работает в сфере международного туризма, предлагая выгодные туры в Таиланд, Турцию, Египет и другие страны мира. Подбор тура и заказ осуществляется в удобном личном кабинете, где можно забронировать отель, купить билеты и оформить путевку без переплат онлайн. Турфирма Pegas работает с проверенными отелями и предлагает широкий выбор направлений и услуг, включая авиабилеты, круизы, трансферы, путевки и подбор лучших отелей для проживания.
Toliknaf
| #
dark market link https://github.com/abacuslink4jjku/abacuslink – darknet websites
WilliamIRrutty
| #
dark web market darknet drug links
OmegaSlayer
| #
Хочу понять в финансах. Что https://primaveracafe.ru посоветуете?
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Работа с нами гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа любого необходимого объема.
С широким ассортиментом редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым текущие трудности превращаются в завтрашние успехи.
Наши товары:
Плоская мишень из иттербия
Rabyitabe
| #
dark market 2025 https://github.com/darkwebsitesyhshv/darkwebsites – bitcoin dark web
GilihrdBuh
| #
На сайте https://avtomastera.net вы найдете различную полезную, актуальную информацию, которая касается ремонта автомобилей. Здесь представлены самые разные рекомендации, которые обязательно помогут мастерам, ответят на многочисленные вопросы, расскажут обо всех нюансах. Вся информация поделена на категории, чтобы было легче и быстрее сориентироваться. Для того чтобы получить доступ ко всем функциям, пройдите регистрацию. Здесь также вы найдете и программы, которые применяются для диагностики.
Kxyufaita
| #
dark web marketplaces darknet drug links
MarkNOlaf
| #
darknet drug store darknet market
FNDaviditabe
| #
dark markets 2025 darknet markets url
CyberWarden
| #
Мемы про финансы: https://oooaleks.ru
DonDonfaita
| #
darknet drug links https://github.com/nexusdarkrtv1u/nexusdark – darknet markets
Pingrutty
| #
dark web market urls https://github.com/aresonioncq0a7/aresonion – dark market
Carltonacets
| #
продвижение сайта заказать продвижение сайта цены
Clubnika Bitcoin
| #
Clubnika Casino is your chance to dive into the exciting world of gambling and win generous prizes.
We offer a wide selection of games, including classic
slots, roulette, blackjack, and unique live dealer games.
Every game at our casino is a chance to win, and security
and fairness are always our top priority.
Why is top casino providers the best choice for gambling players?
In our casino, every player can count on generous bonuses, free spins,
and exclusive offers. Moreover, we ensure fast withdrawals and round-the-clock support, so you can focus on playing.
When is the best time to start playing at Clubnika Casino?
Sign up at Clubnika Casino and get bonuses that
will immediately increase your chances of winning. Here’s what awaits you:
Generous bonuses and free spins for new players.
Promotions and tournaments with big prizes.
Every month we update our game selection, adding new exciting slots
and table games.
Clubnika Casino is the perfect place for those who want to play
and win. https://clubnika-casinonexus.skin/
Waynesailm
| #
Планируете каникулы? купить https://camp-centr.com! Интересные программы, безопасность, забота и яркие эмоции. Бронируйте заранее — количество мест ограничено!
Night37
| #
Эксперты рекомендуют эти ресурсы по финансам: https://rentmegapolis.ru
energoptoru
| #
Выполняем проектирование https://energopto.ru и монтаж всех видов инженерных систем для жилых и коммерческих объектов. Профессиональный подход, сертифицированное оборудование, гарантия качества.
Donaldlaf
| #
darknet markets onion https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet websites
WilliamIRrutty
| #
darknet marketplace dark markets 2025
1win_basr
| #
1вин вход с компьютера https://1win6013.ru/ .
1win_bjml
| #
сайт 1win https://www.knowledge.forum24.ru/?1-0-0-00000101-000-0-0-1742817704 .
1win_woMn
| #
1win скачать kg 1win9109.ru .
mostbet_nyka
| #
мостбет вход http://www.mostbet6004.ru .
Toliknaf
| #
dark market 2025 https://github.com/abacuslink4jjku/abacuslink – dark market url
RobertDaw
| #
Non Gamstop casinos have bonuses that actually pay off—wow!
non gamstop casino in wales
Vortex_Striker
| #
Какие https://ognibarnaula22.ru технологии использовать для финансов?
Kxyufaita
| #
dark markets 2025 darknet drug market
1win_hdsr
| #
1win сайт 1win сайт .
CharlesGes
| #
Играю в онлайн казино ради отдыха, а вы?
https://telegra.ph/Licenzionnoe-kazino-Vavada-i-novye-bonusy-dlya-igrokov-03-27
1win_pkml
| #
1вин про http://knowledge.forum24.ru/?1-0-0-00000101-000-0-0-1742817704/ .
1win_ncMn
| #
1 цшт http://1win9109.ru .
MarkNOlaf
| #
darknet drugs dark web market
mostbet_ozka
| #
mostbet https://mostbet6004.ru .
avtolombardkemerovosig
| #
займ под залог птс
https://complete-jobs.co.uk/employer/ignitionadvertising6458
автоломбард в кемерово под залог
Rabyitabe
| #
darknet markets onion https://github.com/darkwebsitesyhshv/darkwebsites – darkmarket list
SilverZ6
| #
Типичные промахи в финансах: https://dixi59.ru
Pingrutty
| #
darkmarkets https://github.com/aresonioncq0a7/aresonion – darknet market
1win_zysr
| #
1 win kg http://www.1win6013.ru .
1win_ucml
| #
1вин партнерка https://www.knowledge.forum24.ru/?1-0-0-00000101-000-0-0-1742817704 .
1win_ctMn
| #
1вин вход https://1win9109.ru/ .
DonDonfaita
| #
darknet markets links https://github.com/nexusdarkrtv1u/nexusdark – darknet markets onion
mostbet_dxka
| #
мостбет кг мостбет кг .
Vincenttef
| #
бесплатный онлайн генератор QR кодов
WilliamIRrutty
| #
darknet markets onion dark web market urls
DragonSlayer
| #
Обнаружил, что по финансам лучше всего читать здесь: https://sochiperm.ru
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
С широким ассортиментом продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Приступая к взаимодействию с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым сегодняшние вызовы превращаются в завтрашние достижения.
Наши товары:
Керамическая мишень Ta2O5
Vortex_Fang
| #
Особенности реализации финансов: https://kvartned.ru
Felixvah
| #
Проджект Логистикс
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор отборных изделий из редких материалов. Не важно, какие объемы вам необходимы – от мелких партий до обширных поставок, мы гарантируем оперативное исполнение вашего заказа.
Каждая единица товара подтверждена соответствующими документами, подтверждающими их соответствие стандартам. Дружелюбная помощь – наша визитная карточка – мы на связи, чтобы разрешать ваши вопросы по мере того как предоставлять решения под специфику вашего бизнеса.
Доверьте вашу потребность в редких металлах специалистам РедМетСплав и убедитесь в гибкости нашего предложения
Наши товары:
РљСЂСѓРі магниевый РњР›12 – ГОСТ 2856-79 Фольга магниевая РњР›12 – ГОСТ 2856-79 предназначена для использования РІ различных отраслях, таких как электроника, машиностроение Рё строительство. Рта фольга обладает высокой прочностью Рё легкостью, что делает ее идеальным материалом для создания защитных покрытий Рё теплоизоляции. Приобретая данный РїСЂРѕРґСѓРєС‚, РІС‹ обеспечиваете высокое качество Рё надежность. Если РІС‹ хотите улучшить СЃРІРѕРё производственные процессы, купить Фольга магниевая РњР›12 – ГОСТ 2856-79 будет правильным решением. РњС‹ гарантируем быструю доставку Рё отличное обслуживание.
Donaldlaf
| #
darknet market https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet market
Kxyufaita
| #
tor drug market darkmarket 2025
FNDaviditabe
| #
darknet markets url dark markets 2025
MarkNOlaf
| #
dark web drug marketplace dark web market links
Toliknaf
| #
darknet market lists https://github.com/abacuslink4jjku/abacuslink – dark market 2025
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для оформления товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с непревзойденным обслуживанием.
Наша команда поддержки всегда на связи, чтобы помочь вам в выборе товаров и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете надежность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – идеальный вариант для вас.
поставляемая продукция:
Медная капиллярная трубка 1.7х0.55 мм М2р ГОСТ 2624-2016 Покупайте медную капиллярную трубку у нас для идеального теплоотвода и надежности. Широкий выбор размеров и конфигураций для различных проектов. Отличная теплопроводность и гибкость монтажа, гарантирующие долговечность и экономию времени и денег.
Pingrutty
| #
tor drug market https://github.com/aresonioncq0a7/aresonion – darknet drugs
Vincenttef
| #
https://intekey.ru/
Rabyitabe
| #
dark websites https://github.com/darkwebsitesyhshv/darkwebsites – dark market
Blue_Code
| #
Смешные случаи в финансах: https://pik-leasing.ru
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Закупка у нас гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Имея широкий спектр продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым текущие трудности превращаются в завтрашние достижения.
Наши товары:
Мишень для распыления палладия (Pd, 3N5, плоская)
Dragon_Slayer
| #
Факты и вымысел о https://gik-invest.ru финансах.
WilliamIRrutty
| #
darknet marketplace tor drug market
Vincenttef
| #
бесплатный онлайн генератор QR кодов
DonDonfaita
| #
darkmarket 2025 https://github.com/nexusdarkrtv1u/nexusdark – dark markets
RobertDaw
| #
Non-Gamstop casino sites feel so much more relaxed—big thumbs up!
non.gamstop casino
Kxyufaita
| #
darknet drugs dark websites
FNDaviditabe
| #
onion dark website darknet drug store
MarkNOlaf
| #
dark web market darknet site
Vincenttef
| #
Создание QR кодов
avtolombardmsksig
| #
взять займ под залог птс
https://www.behavioralhealthjobs.com/companies/avtolombard-zalog-invest1534/
автоломбард авто
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Закупка у нас гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Обладая обширной линейкой редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым текущие трудности превращаются в завтрашние достижения.
Наши товары:
Металлическая мишень для распыления
Vortex20
| #
Что нельзя делать с финансами? https://rospremierinvest24.ru
Donaldlaf
| #
dark websites https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet markets 2025
MuwozSmomb
| #
Официальный партнер окон REHAU https://okna-intex.ru/ это возможность заказать по выгодным ценам окна с установкой под ключ. Ознакомьтесь на сайте со всеми преимуществами окон Рехау или рассчитайте стоимость окон по доступной цене. Мы предлагаем широкий выбор оконных систем, включая пластиковые и алюминиевые окна. Наши окна отличаются высоким качеством, долговечностью и надежностью.
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Закупка у нас гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
Имея широкий спектр продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым сегодняшние вызовы превращаются в завтрашние успехи.
Наша продукция:
Плоская мишень для распыления свинца 4N
Pingrutty
| #
darkmarket 2025 https://github.com/aresonioncq0a7/aresonion – darknet drug market
Toliknaf
| #
dark market onion https://github.com/abacuslink4jjku/abacuslink – dark web markets
CharlesGes
| #
Играю в онлайн казино ради кайфа, а вы?
https://telegra.ph/Luchshie-live-igry-nedeli-v-kazino-Mostbet-03-27
Red_Nomad
| #
Подробный https://kpk-agrorostorg-russia.ru разбор финансов.
WilliamIRrutty
| #
dark web markets darkmarket
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Закупка у нас гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа требуемого количества.
Обладая обширной линейкой продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым сегодняшние вызовы превращаются в завтрашние достижения.
Наши товары:
Гранулы магния 3N5
Vincenttef
| #
https://orgnaztech.mirtesen.ru/blog/43202871346/Gde-Kazhdyiy-Gost-Osobennyiy-Otel-Glory
Rabyitabe
| #
darknet market links https://github.com/darkwebsitesyhshv/darkwebsites – darknet markets url
BlueSlayer
| #
Как сэкономить на финансах: https://lombardveka.ru.
Kxyufaita
| #
dark web drug marketplace darknet markets
MarkNOlaf
| #
darknet market lists dark web link
FNDaviditabe
| #
darknet drug market darknet drug market
CharlesGes
| #
Онлайн казино с VIP-программой — чувствую себя особенным!
https://telegra.ph/Mostbet-kazino-mify-o-live-igrah-top-5-razoblachenij-03-27
Vincenttef
| #
Glory Casino app
DonDonfaita
| #
dark market https://github.com/nexusdarkrtv1u/nexusdark – dark markets 2025
Edwardignok
| #
На берегу моря нашли русалку https://x.com/Fariz418740/status/1905487137991958614
Vincenttef
| #
автоматизация бизнес-процессов
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог качественных изделий из редких материалов. Не важно, какие объемы вам необходимы – от мелких партий до крупных поставок, мы гарантируем быстрое выполнение вашего заказа.
Каждая единица изделия подтверждена соответствующими документами, подтверждающими их происхождение. Превосходное обслуживание – наша визитная карточка – мы на связи, чтобы ответить на ваши вопросы а также предоставлять решения под специфику вашего бизнеса.
Доверьте потребности вашего бизнеса профессионалам РедМетСплав и убедитесь в широком спектре предлагаемых возможностей
Наша продукция:
РљСЂСѓРі ниобиевый РќР¦-2 РљСЂСѓРі ниобиевый РќР¦-2 – это высококачественный РїСЂРѕРґСѓРєС‚, изготовленный РёР· чистого РЅРёРѕР±РёСЏ, который обладает превосходными физико-химическими свойствами. Применяется РІ различных областях, включая авиацию Рё ядерную энергетику. Р—Р° счет своей стойкости Рє РєРѕСЂСЂРѕР·РёРё, РєСЂСѓРі обеспечивает долговечность Рё надежность РІ эксплуатации. Р’С‹ можете купить РљСЂСѓРі ниобиевый РќР¦-2 для оптимизации процессов обработки материалов. РќРµ упустите возможность повысить эффективность своего производства СЃ помощью этого уникального продукта.
Vincenttef
| #
Лучший генератор QR-кодов
Demetra
| #
Genom att ange din e-post bekräftar du att har läst igenom och accepterat vår integritets- och cookiepolicy.
Pingrutty
| #
darkmarket list https://github.com/aresonioncq0a7/aresonion – dark web sites
NightV4
| #
Свежие тренды в https://msk124.ru финансах.
Donaldlaf
| #
darknet drug links https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – onion dark website
WilliamIRrutty
| #
dark market list dark market list
WolfHacker
| #
Основы https://bogner-vision.ru финансов для новичков.
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы обеспечить любой запрос с прекрасным качеством обслуживания.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в подборе нужных изделий и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете надежность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – идеальный вариант для вас.
Наши товары:
Полоса РёР· магнитно-РјСЏРіРєРёС… сплавов 1.2 РјРј 40РќРљРњ ГОСТ 10160-75 РЁРёСЂРѕРєРёР№ выбор полос РёР· магнитно-РјСЏРіРєРёС… сплавов для электротехнических устройств. Низкая коерцитивная сила Рё превосходная магнитная проницаемость. Рдеально для энергетики, силовой электроники Рё медицинского оборудования. Коррозионная устойчивость обеспечивает долгий СЃСЂРѕРє службы.
ArthurTog
| #
https://github.com/ms-core/Microsoft-Project/releases
Vincenttef
| #
https://orgnaztech.mirtesen.ru/blog/43202871346/Gde-Kazhdyiy-Gost-Osobennyiy-Otel-Glory
Gefevpek
| #
Visit https://bestcasinoideal.com/ and discover expert reviews, top strategies and essential tips. Explore online slots, table games, live dealer experiences. We have compiled a complete collection of the best online casinos and gambling sites for 2025, tested and rated by experts.
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Закупка у нас гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа требуемого количества.
С широким ассортиментом редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым текущие трудности превращаются в завтрашние успехи.
Наша продукция:
Керамическая мишень для распыления Р±РѕСЂР° – плоская
Toliknaf
| #
darkmarket 2025 https://github.com/abacuslink4jjku/abacuslink – darknet drug market
Kxyufaita
| #
dark market list dark web market
FNDaviditabe
| #
dark web markets darknet market lists
Rabyitabe
| #
darknet market list https://github.com/darkwebsitesyhshv/darkwebsites – darknet market lists
avtolombardmsksig
| #
деньги под птс круглосуточные
https://www.iratechsolutions.com/employer/thunder-consulting1245/
займ под залог отечественного авто
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор качественных изделий из ценных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до масштабных поставок, мы обеспечиваем своевременную реализацию вашего заказа.
Каждая единица продукции подтверждена требуемыми документами, подтверждающими их качество. Превосходное обслуживание – то, чем мы гордимся – мы на связи, чтобы ответить на ваши вопросы и находить ответы под специфику вашего бизнеса.
Доверьте ваш запрос профессионалам РедМетСплав и убедитесь в гибкости нашего предложения
Наша продукция:
Проволока магниевая G-MgAl6 – DIN 1729 Part 2 Полоса магниевая G-MgAl6 – DIN 1729 Part 2 представляет СЃРѕР±РѕР№ высококачественный материал, который идеально РїРѕРґС…РѕРґРёС‚ для различных промышленных применений. Благодаря легкости Рё прочности магний используется РІ автомобильной Рё авиационной отраслях. Полоса обладает отличной РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ стойкостью, что делает ее надежной РІ условиях повышенной влажности. Приобретая данную полосу, РІС‹ получаете РїСЂРѕРґСѓРєС‚, соответствующий всем стандартам качества. РќРµ упустите возможность улучшить СЃРІРѕРё проекты, купите Полоса магниевая G-MgAl6 – DIN 1729 Part 2 уже сегодня!
Blade7286
| #
Как считаете, где лучше всего https://bonami-salon.ru понять финансы?
Donaldnuh
| #
кайт египет
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Закупка у нас гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
Обладая обширной линейкой продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие всем нормам.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым сегодняшние вызовы превращаются в завтрашние успехи.
Наши товары:
Керамическая мишень Титаната Бария
Edwardignok
| #
На берегу моря нашли русалку https://x.com/Fariz418740/status/1905487137991958614
DonDonfaita
| #
darkmarket url https://github.com/nexusdarkrtv1u/nexusdark – darknet markets
JoshuaUsady
| #
настольные игры Table Mania – магазины настольных игр, в котором представлен широчайший ассортимент настольных игр. У нас широко представлены как семейные, детские и вечериночные, так и сложные стратегические, коллекционно-карточные и тактические игры с миниатюрами.
CyberSentinel
| #
По данным аналитиков, лучший источник о финансах – https://gorod-zaimov.ru
Pingrutty
| #
dark market onion https://github.com/aresonioncq0a7/aresonion – dark web markets
WilliamIRrutty
| #
bitcoin dark web darknet links
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем внушительный выбор продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с непревзойденным обслуживанием.
Наша команда поддержки всегда на связи, чтобы помочь вам в выборе товаров и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете надежность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – ваш лучший выбор.
Наши товары:
Полоса РёР· магнитно-РјСЏРіРєРёС… сплавов 0.7 РјРј 50РќРџ ГОСТ 10160-75 РЁРёСЂРѕРєРёР№ выбор полос РёР· магнитно-РјСЏРіРєРёС… сплавов для электротехнических устройств. Низкая коерцитивная сила Рё превосходная магнитная проницаемость. Рдеально для энергетики, силовой электроники Рё медицинского оборудования. Коррозионная устойчивость обеспечивает долгий СЃСЂРѕРє службы.
Donaldlaf
| #
onion dark website https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet drug store
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор качественных изделий из ценных материалов. Не важно, какие объемы вам необходимы – от мелких партий до масштабных поставок, мы обеспечиваем своевременную реализацию вашего заказа.
Каждая единица товара подтверждена всеми необходимыми документами, подтверждающими их качество. Превосходное обслуживание – наш стандарт – мы на связи, чтобы улаживать ваши вопросы а также адаптировать решения под требования вашего бизнеса.
Доверьте потребности вашего бизнеса специалистам РедМетСплав и убедитесь в широком спектре предлагаемых возможностей
Наша продукция:
Пруток магниевый B 92 (9980A) – ASTM B92 Проволока магниевая B 92 (9980A) – ASTM B92 предлагает высококачественный материал для производства Рё изготовления серьезных изделий. РћРЅР° обеспечивает надежность Рё долговечность, что делает её идеальным выбором для различных промышленных применений. Благодаря СЃРІРѕРёРј отличным свойствам, этот РїСЂРѕРґСѓРєС‚ легко обрабатывается Рё сваривается.Покупая проволоку магниевую B 92 (9980A) – ASTM B92, РІС‹ получаете РЅРµ только надежный материал, РЅРѕ Рё уверенность РІ качестве своей продукции. РќРµ упустите возможность купить Проволока магниевая B 92 (9980A) – ASTM B92 Рё повысить эффективность СЃРІРѕРёС… проектов.
FNDaviditabe
| #
dark web market urls dark web marketplaces
MarkNOlaf
| #
darknet markets url darknet markets 2025
Kxyufaita
| #
darknet site darknet links
Pioneer2884
| #
Особенности https://sunandmoonsaransk.ru реализации финансов.
Billydrula
| #
elonbet casino BD
Toliknaf
| #
darknet markets url https://github.com/abacuslink4jjku/abacuslink – darknet markets url
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор качественных изделий из редких материалов. Не важно, какие объемы вам необходимы – от небольших закупок до обширных поставок, мы обеспечиваем быстрое выполнение вашего заказа.
Каждая единица продукции подтверждена всеми необходимыми документами, подтверждающими их качество. Опытная поддержка – то, чем мы гордимся – мы на связи, чтобы улаживать ваши вопросы а также адаптировать решения под особенности вашего бизнеса.
Доверьте ваш запрос профессионалам РедМетСплав и убедитесь в множестве наших преимуществ
Наша продукция:
Рзделия РёР· кобальта Wallex 100 Рзделия РёР· кобальта Wallex 100 идеально РїРѕРґС…РѕРґСЏС‚ для профессионалов, ценящих качество Рё надежность. Рти высококачественные материалы обеспечивают отличную износостойкость Рё долговечность. РћРЅРё применяются РІ различных областях, таких как промышленность Рё медицина. Рнновационные технологии производства гарантируют безопасность Рё эффективность. Каждый РїСЂРѕРґСѓРєС‚ РїСЂРѕС…РѕРґРёС‚ строгий контроль. РќРµ упустите шанс купить Рзделия РёР· кобальта Wallex 100 Рё обеспечить себя надежными решениями для будущих задач. Сделайте выбор РІ пользу долговечности Рё качества.
MetaMask Download
| #
MetaMask Download is super quick. The wallet’s design is simple yet powerful, making it ideal for beginners and experts alike.
Billydrula
| #
elonbet casino
MichaelHab
| #
esitella to doslownie tragedia! Tresci nieaktualne, a w niektorych przypadkach zupelnie nieprawidlowe. Probowalem dowiedziec sie wazne tresci o zdrowiu, ale natrafilem na mnostwo bledow i jawnych absurdow. Mozna pomyslec, ze tresci redagowane przez ludzi bez wiedzy! To jest niebezpieczne dla uzytkownikow, ktorzy ufaja tym danym. Wlasciciele nie odpowiadaja usterek, a opinie z duzym prawdopodobienstwem sfalszowane.
EdwardBiorn
| #
Курьер привёз вовремя, упаковка целая!
женский костюм купить томск
Davidpocky
| #
kraken – кракен даркнет, kra30 at
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф гарантирует все стадии сделки, предоставляя полный пакет необходимых документов для оформления товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы поставить любой запрос с прекрасным качеством обслуживания.
Наша команда поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – идеальный вариант для вас.
Наша продукция:
Медный переходной пресс тройник 64х25 мм М2РМ ГОСТ Р52948-2008 Приобретайте надежные медные переходные тройники для соединения труб с разным диаметром. Устойчивы к коррозии, легки в монтаже и обеспечивают надежное соединение. Широкий выбор размеров. Гарантированное качество и долговечность.
Rabyitabe
| #
dark web sites https://github.com/darkwebsitesyhshv/darkwebsites – darknet markets links
VortexX9
| #
Практическое применение финансов: https://ipoteka-kurgan.ru
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор отборных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от мелких партий до масштабных поставок, мы гарантируем своевременную реализацию вашего заказа.
Каждая единица продукции подтверждена соответствующими документами, подтверждающими их происхождение. Превосходное обслуживание – наша визитная карточка – мы на связи, чтобы разрешать ваши вопросы а также адаптировать решения под специфику вашего бизнеса.
Доверьте потребности вашего бизнеса профессионалам РедМетСплав и убедитесь в множестве наших преимуществ
поставляемая продукция:
Рзделия РёР· кобальта РРџ482 Рзделия РёР· кобальта РРџ482 представляют СЃРѕР±РѕР№ высококачественные изделия, обладающие долговечностью Рё устойчивостью Рє РєРѕСЂСЂРѕР·РёРё. РћРЅРё идеально РїРѕРґС…РѕРґСЏС‚ для применения РІ условиях высокой температуры Рё механических нагрузок. Каждый РїСЂРѕРґСѓРєС‚ РїСЂРѕС…РѕРґРёС‚ строгий контроль качества, что гарантирует надежность Рё долговечность. Корпоративные клиенты Рё профессионалы предпочитают нашу продукцию Р·Р° ее высокую производительность Рё эффективность. Если РІС‹ хотите увеличить производительность вашего оборудования, купить Рзделия РёР· кобальта РРџ482 — это отличный выбор. Обратите внимание РЅР° РёС… универсальность Рё превосходные эксплуатационные характеристики.
RockyMah
| #
Planning to transfer cash in the popular Aviator game?
We’ve got you covered.
First, log in, then navigate to the top-up area.
Now, select your most convenient payment option:
e-wallet.
Input the sum you want to transfer — as much as you want.
Approve the payment and you’re all set to play!
For more info, visit http://cajnews.co.ke/ — no redirects.
No delays — hit the skies with confidence!
WilliamIRrutty
| #
darknet markets links dark web sites
Pingrutty
| #
dark web drug marketplace https://github.com/aresonioncq0a7/aresonion – darknet market links
BrianBoofs
| #
https://www.radiofrance.fr/franceinter/podcasts/lumieres-dans-la-nuit/lumieres-dans-la-nuit-du-mardi-09-juin-2020-8311597
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с непревзойденным обслуживанием.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в подборе нужных изделий и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – наилучшее решение для вашего бизнеса.
поставляемая продукция:
РўСЂСѓР±Р° РёР· драгоценных металлов палладиевая 500С…1С…0.7 РјРј РџРґР82-18 РўРЈ Гарантированное качество Рё изысканный дизайн – купите драгоценные трубы для ювелирных украшений, машиностроения Рё архитектуры. РЁРёСЂРѕРєРёР№ выбор Рё высокое качество! Доставка РїРѕ Р РѕСЃСЃРёРё.
1win_veSt
| #
wan win http://belbeer.borda.ru/?1-6-0-00001583-000-0-0/ .
Michaeldab
| #
семяныч официальный сайт купить семена
mostbet_kner
| #
мрстбет мрстбет .
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Имея широкий спектр редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие всем нормам.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит надежного партнера, с которым сегодняшние вызовы превращаются в завтрашние успехи.
Наши товары:
Гранулы циркония
DonDonfaita
| #
dark markets 2025 https://github.com/nexusdarkrtv1u/nexusdark – bitcoin dark web
Guardian1224
| #
Видел прогнозы, что Финансы будет главным трендом. Возражения? https://treid-leasing.ru
1win_wjkt
| #
1win rossvya https://1win6018.ru/ .
Billydrula
| #
elonbet casino BD
Kxyufaita
| #
darknet websites dark markets 2025
1win_mmSt
| #
игра 1вин игра 1вин .
FNDaviditabe
| #
dark market onion dark website
MarkNOlaf
| #
dark websites dark web market links
mostbet_user
| #
mostbet kg скачать на андроид http://www.girikms.forum24.ru/?1-1-0-00000361-000-0-0-1742819287 .
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф гарантирует все стадии сделки, предоставляя полный пакет необходимых документов для оформления товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы поставить любой запрос с высоким уровнем сервиса.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете достоверность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – идеальный вариант для вас.
Наша продукция:
Лента СЃ заданными свойствами упругости 0.35С…250 РјРј 44РќРҐРўР® ГОСТ 14117-85 Лента СЃ заданными свойствами упругости предлагает высококачественные материалы, определенные параметры упругости Рё широкий выбор размеров для надежного Рё РіРёР±РєРѕРіРѕ крепления. Рспользуется РІ строительстве, производстве Рё машиностроении. Обеспечивает легкость установки, надежность, долговечность Рё адаптацию Рє различным техническим требованиям.
mostbet_jwSn
| #
мост бет https://www.kharkovbynight.forum24.ru/?1-15-0-00003047-000-0-0 .
1win_ankt
| #
ваучер 1win ваучер 1win .
Donaldlaf
| #
darknet market links https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darkmarket list
Billydrula
| #
elonbet casino
mostbet_hiSn
| #
мос бет мос бет .
1win_ovSt
| #
1vin https://belbeer.borda.ru/?1-6-0-00001583-000-0-0 .
Toliknaf
| #
darkmarkets https://github.com/abacuslink4jjku/abacuslink – dark web marketplaces
mostbet_ncer
| #
скачат мостбет скачат мостбет .
Phantom922
| #
Пытаюсь разобраться в финансах. Что посоветуете? https://avtolombard-13.ru
Vincenttef
| #
https://intekey.ru/
Pingrutty
| #
dark web sites https://github.com/aresonioncq0a7/aresonion – darknet markets url
Billydrula
| #
elonbet casino BD
OmegaFang
| #
Примеры успеха https://detektiv-ufa.ru в финансах.
1win_tgkt
| #
бк 1win https://1win6018.ru/ .
mostbet_qkSn
| #
мостбет chrono http://kharkovbynight.forum24.ru/?1-15-0-00003047-000-0-0 .
Rabyitabe
| #
darknet site https://github.com/darkwebsitesyhshv/darkwebsites – darknet market
WilliamIRrutty
| #
dark markets darknet markets url
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор высококачественных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от мелких партий до масштабных поставок, мы гарантируем своевременную реализацию вашего заказа.
Каждая единица продукции подтверждена всеми необходимыми документами, подтверждающими их соответствие стандартам. Превосходное обслуживание – наша визитная карточка – мы на связи, чтобы улаживать ваши вопросы по мере того как находить ответы под особенности вашего бизнеса.
Доверьте ваш запрос специалистам РедМетСплав и убедитесь в множестве наших преимуществ
Наши товары:
Рзделия РёР· титана Р’Рў20 РџРѕРєРѕРІРєР° титановая Р’Рў20 — это высококачественный РїСЂРѕРґСѓРєС‚, обладающий прочностью Рё легкостью. Рспользуется РІ авиационной, космической Рё медицинской отраслях. Его уникальные свойства обеспечивают отличные характеристики устойчивости Рє РєРѕСЂСЂРѕР·РёРё Рё высокой температуре. Применение данной РїРѕРєРѕРІРєРё позволяет значительно увеличить надежность изделий. Чтобы обеспечить качественное выполнение проектов, стоит подумать, РіРґРµ купить РџРѕРєРѕРІРєР° титановая Р’Рў20. Выбор данной марки титанового сплава — это гарантированный успех РІ вашем бизнесе. РќРµ упустите возможность повысить качество ваших товаров!
DonDonfaita
| #
dark web market links https://github.com/nexusdarkrtv1u/nexusdark – dark market
Vincenttef
| #
бесплатный онлайн генератор QR кодов
KennethTyday
| #
Печать рекламных буклетов https://tipografiya-buklety.ru ярко, качественно, профессионально. Форматы A4, евро, индивидуальные размеры. Работаем с частными и корпоративными заказами.
riobet
| #
рио бет риобет вход на сайт
FNDaviditabe
| #
darknet markets onion address dark web sites
Vincenttef
| #
https://intekey.ru/
Kxyufaita
| #
dark web market dark web market urls
GhostProtocol
| #
Типичные промахи в финансах: https://viessmann72.ru
MarkNOlaf
| #
dark market url darkmarket 2025
Omega_Hunter
| #
Изучите этот материал https://gk-cent.ru о финансах.
Donaldlaf
| #
best darknet markets https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet markets links
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы поставить любой запрос с высоким уровнем сервиса.
Наша команда поддержки всегда на связи, чтобы помочь вам в подборе нужных изделий и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – идеальный вариант для вас.
поставляемая продукция:
Медный стандартный двухраструбный отвод 90 градусов под пайку 108х92х2.1 мм 47.5х51.5 мм мягкая пайка М3т ГОСТ 32590-2013 Покупайте медные двухраструбные отводы 90 градусов под пайку на Редметсплав.рф. Надежные и долговечные отводы из высококачественной меди для систем водоснабжения, отопления и кондиционирования воздуха. Гарантированно надежные соединения, простой монтаж и оптимальное распределение потока.
Pingrutty
| #
darknet drug links https://github.com/aresonioncq0a7/aresonion – dark market list
Toliknaf
| #
darknet links https://github.com/abacuslink4jjku/abacuslink – darknet market lists
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор высококачественных изделий из ценных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до крупных поставок, мы гарантируем своевременную реализацию вашего заказа.
Каждая единица товара подтверждена всеми необходимыми документами, подтверждающими их соответствие стандартам. Дружелюбная помощь – наш стандарт – мы на связи, чтобы улаживать ваши вопросы и адаптировать решения под особенности вашего бизнеса.
Доверьте ваш запрос профессионалам РедМетСплав и убедитесь в множестве наших преимуществ
Наши товары:
Проволока вольфрамовая Р’Рђ Проволока вольфрамовая Р’Рђ является РѕРґРЅРёРј РёР· важнейших материалов РІ металлургии Рё промышленности. РћРЅР° обладает высокой температурной стойкостью Рё прочностью, что делает её идеальным выбором для различных технологий, включая сварку Рё электронику. Благодаря СЃРІРѕРёРј уникальным свойствам, Проволока вольфрамовая Р’Рђ широко используется РІ производстве ламп Рё РґСЂСѓРіРёС… высокотемпературных устройств.Если РІС‹ ищете надежный материал, который отвечает современным стандартам качества Рё эффективности, вам стоит купить Проволока вольфрамовая Р’Рђ. Ртот РїСЂРѕРґСѓРєС‚ гарантирует долговечность Рё стабильную работу РІ самых сложных условиях.
Vincenttef
| #
https://orgnaztech.mirtesen.ru/blog/43202871346/Gde-Kazhdyiy-Gost-Osobennyiy-Otel-Glory
Rabyitabe
| #
dark web market links https://github.com/darkwebsitesyhshv/darkwebsites – darknet marketplace
Billydrula
| #
elonbet casino
WilliamIRrutty
| #
dark market darknet markets
Wolf950
| #
Ответы на частые вопросы о финансах: https://ooo-autolombard-88.ru
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем внушительный выбор продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф гарантирует все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы поставить любой запрос с прекрасным качеством обслуживания.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в выборе товаров и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете надежность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – идеальный вариант для вас.
поставляемая продукция:
Медный отвод пресс 45 градусов 34 мм М2РМ ГОСТ Р52948-2008 Приобретите высококачественные медные отводы пресс для систем водоснабжения и отопления. Надежное соединение, устойчивость к коррозии, простота монтажа. Широкий ассортимент. Доставка по России.
DonDonfaita
| #
darknet drug links https://github.com/nexusdarkrtv1u/nexusdark – dark web sites
Vincenttef
| #
https://orgnaztech.mirtesen.ru/blog/43202871346/Gde-Kazhdyiy-Gost-Osobennyiy-Otel-Glory
DarkV4
| #
Кто-нибудь разбирается в финансах? Куда зайти? https://udvorperm.ru
FNDaviditabe
| #
dark web drug marketplace bitcoin dark web
Kxyufaita
| #
dark web market urls darkmarket 2025
avtolombardnovosibsig
| #
кредит под птс
http://webheaydemo.co.uk/profile/rolandomahler
займ под залог кредитного авто
MarkNOlaf
| #
darknet market list darknet site
Pingrutty
| #
darkmarket link https://github.com/aresonioncq0a7/aresonion – dark web market
pricelcaree
| #
Подробнее в блоге обзоров прицелов и другой оптической техники на сайте leupold-optic.ru ссылка
Donaldlaf
| #
darknet markets 2025 https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet drug store
Vincenttef
| #
https://orgnaztech.mirtesen.ru/blog/43202871346/Gde-Kazhdyiy-Gost-Osobennyiy-Otel-Glory
Vincenttef
| #
https://orgnaztech.mirtesen.ru/blog/43202871346/Gde-Kazhdyiy-Gost-Osobennyiy-Otel-Glory
Toliknaf
| #
darknet drug market https://github.com/abacuslink4jjku/abacuslink – best darknet markets
Blade9578
| #
Предлагаю подискутировать о https://comfort-textile.ru финансах.
Vincenttef
| #
Создание QR кодов
WilliamIRrutty
| #
dark web markets dark web market links
DriverBaile
| #
Соседи по дому посоветовали Городскую Ритуальную Службу. Организация похорон была выполнена безупречно. Благодарим за поддержку.
Volodyanaf
| #
darknet links darknet links
Rabyitabe
| #
bitcoin dark web https://github.com/darkwebsitesyhshv/darkwebsites – dark markets
Billydrula
| #
elonbet casino
Vuxerelayes
| #
На сайте https://remont-okon-63.ru/ уточните номер телефона для того, чтобы воспользоваться такой нужной услугой, как ремонт, регулировка окон независимо от сложности. Прямо сейчас вы сможете воспользоваться возможностью вызвать мастера. Для этого следует указать номер телефона, а также имя. Среди основных услуг, которые оказываются в этой компании, выделяют: ремонт балконных дверей, производство москитных сеток, стеклопакетов. Кроме того, доступен и срочный ремонт конструкций. Все работы выполняются в соответствии с требованиями.
Larrytwicy
| #
popular steroid brands https://anabolshop.org/
Code7907
| #
Что нового в финансах? https://broker86.ru.
Vincenttef
| #
бесплатный онлайн генератор QR кодов
Michaelesoke
| #
Enhance your individual auto with our own tuning services. Change your car into a unique masterpiece. Our team of engineers is dedicated to supplying top-quality results that outperform your wishes. Whether you’re looking to increase performance, revamp aesthetics, or integrate new features, we have the knowledge to deliver your vision to life. Visit our website at https://timbrellosautobody.com/ to explore our services and schedule a consultation today.Let us assist you construct the machine of your dreams.
Kxyufaita
| #
dark market list darknet markets links
Everettsox
| #
анализ на наркотики купить analiz-kupit-spb.ru
Billydrula
| #
elonbet casino
DonDonfaita
| #
darknet drugs https://github.com/nexusdarkrtv1u/nexusdark – dark markets 2025
Billydrula
| #
elonbet casino BD
Pingrutty
| #
dark web markets https://github.com/aresonioncq0a7/aresonion – darknet drug market
Billydrula
| #
elonbet casino BD
ThunderProtocol
| #
Исследование финансов: https://gzhc38.ru
Donaldlaf
| #
darknet marketplace https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet sites
deheTus
| #
Компания A.X.O.N предлагает квалифицированные услуги. Создаем для бизнеса AI-решения. Работаем с юридическими и физическими лицами по всему миру. Формат и условия оплаты обсуждаются персонально под ваш запрос. https://axonbusiness.ru – тут представлены ответы на популярные вопросы. Вы сможете узнать, какие можно автоматизировать задачи. Мы выстраиваем бизнес-логику, настраиваем процессы и доводим до результата. Всегда на связи и готовы ответить на интересующие вопросы. Давайте начнем с консультации, это бесплатно.
edacaree
| #
Доставка продуктов и готовой еды на дом – EdaDostavkoy.ru здесь
WilliamIRrutty
| #
darknet market links darknet market lists
Toliknaf
| #
dark market list https://github.com/abacuslink4jjku/abacuslink – darknet marketplace
Natasesunice
| #
Ищете оригинальный мерч от любимых российских и зарубежных звезд, фестивалей и шоу? Посетите https://showstaff.ru/ – мы с 2002 года радуем наших клиентов качественной продукцией, доставкой по всей России и поддержкой талантливых артистов. Заходите на наш сайт и выбирайте свой стиль! ShowStaff – это магазин оригинального мерча, официально и качественно. Посетите каталог, и вы обязательно найдете для себя уникальные вещи!
Billydrula
| #
elonbet casino BD
Bevaxowreave
| #
На сайте https://smartflow.ru вы сможете выбрать качественную, функциональную сантехнику для обустройства ванной комнаты в доме либо квартире. Ассортимент «SMARTFLOW» включает в себя такие изделия, которые выполнены из высококачественных и современных материалов, безупречного стиля. Все унитазы, биде, модульные ванные идеально впишутся в концепцию. Каждая вещь идеально сочетает надежность, прочность, а также изысканность. Для того чтобы выбрать что-то определенное, изучите галерею, ознакомьтесь и с особой системой смыва «Торнадо».
WolfZ4
| #
Согласно исследованиям, лучший источник о финансах – https://kpkkc-matkap.ru
maslyanie transformatori_mbPi
| #
тмг трансформатор тмг трансформатор .
programma 1s_temr
| #
1с бухгалтерия 8 купить 1с бухгалтерия 8 купить .
programma 1s_ydka
| #
покупка 1с покупка 1с .
Billydrula
| #
elonbet casino
Rabyitabe
| #
bitcoin dark web https://github.com/darkwebsitesyhshv/darkwebsites – darkmarket link
Shadow464
| #
Памятка для работы с финансами: https://webresurs-72.ru
cawuomisk
| #
Компания АБК предлагает аренду бытовок, которые производятся из высококачественных материалов. Ассортимент выбора контейнеров большой, цены приемлемые. Процесс аренды понятен и прост, сотрудники внимательны к пожеланиям, они профессионалы своего дела. Ищете аренда блок контейнеров? Arendabk.ru – портал, где имеются отзывы и фотогалерея. Предлагаем в аренду исключительно достойные контейнеры. Доставим их в минимальные сроки. С радостью на все вопросы ответим, и верный выбор поможем сделать. Для нас важно, чтобы вам было приятно с нами работать.
FNDaviditabe
| #
darknet drug market dark market list
Pingrutty
| #
darknet websites https://github.com/aresonioncq0a7/aresonion – darknet site
MarkNOlaf
| #
darkmarkets darknet site
Cazrwjj
| #
Привет!
Без ВУЗа очень нелегко было продвигаться по карьерной лестнице. Сегодня же этот документ не дает совершенно никаких гарантий, что получится найти хорошую работу. Намного более важны профессиональные навыки и знания специалиста, а также его опыт работы. По этой причине решение о заказе диплома стоит считать рациональным. Купить диплом о высшем образовании tweakbook.us/read-blog/1210_diplom-moskva.html
zaimpodzalogptsekbsig
| #
кредит под птс автомобиля
avtolombard-pid-zalog-pts.ru/ekb.html
деньги под залог птс быстро
WilliamDix
| #
Мы изготавливаем дипломы любой профессии по выгодным ценам. Дипломы производят на настоящих бланках государственного образца Заказать диплом института diplomidlarf.ru
Lazrglf
| #
Где купить диплом по необходимой специальности?
Купить диплом института по невысокой стоимости можно, обращаясь к надежной специализированной фирме.: diplommy.ru
DonDonfaita
| #
darknet markets url https://github.com/nexusdarkrtv1u/nexusdark – darknet markets links
Volodyanaf
| #
dark web link dark web markets
asgdh_fiche
| #
Do not buy anything from them!!!
DannyTox
| #
Большой выбор из 8677 наименований на складе. В розницу по оптовые ценам от производителя. Доступная доставка в Москву курьерской службой до 2х дней. Фабричное производство: купить сигареты в москве
Sazrbyo
| #
купить диплом в садик
Sazrjeh
| #
Привет!
Приобрести диплом института по невысокой стоимости возможно, обращаясь к проверенной специализированной фирме. Заказать диплом: diplomus-spb.ru/kupit-diplom-spetsialista-7/
KennethHew
| #
Как выбрать надёжное оборудование для майнинга в 2025 году — Узнайте подробности здесь https://www.buzzbii.com/Cryptons
Donaldlaf
| #
dark market onion https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – darknet markets onion address
xoxetiProva
| #
Caspian Training Group преподаватели компании в своей работе толк знают. Они постоянно проходят курсы повышения квалификации. Вас персональный подход компании порадует. https://xn—-7sbecpcasfm0beeaecirc5b3a2g.kz/ – тут более детальная информация о нас представлена, посмотрите ее в любое время. Caspian Training Group помогает компаниям успешно реализовывать проекты корпоративного обучения. Результаты ваши ожидания превзойдут. Сотрудники станут увереннее в своей работе и улучшат навыки взаимодействия в команде.
WilliamIRrutty
| #
dark markets 2025 darknet market lists
Reaper1247
| #
Конкретные https://rps63.ru шаги для работы с финансами.
SejehewTot
| #
Welcome to the official Joy Casino blog https://officialjoycasino.net/ — a place where you can explore the fascinating world of online gambling from different perspectives: game mechanics, psychology, responsible gaming and industry analytics. If you are looking for a deep dive into gaming strategies, casino technology or responsible gaming tips, you have come to the right place.
Silver904
| #
Как войти в тему финансов? https://ed-centr.ru
Toliknaf
| #
darkmarket link https://github.com/abacuslink4jjku/abacuslink – darknet market
Billydrula
| #
elonbet casino BD
Xazrkae
| #
Купить диплом о высшем образовании!
Мы предлагаем дипломы любой профессии по приятным ценам. Вы заказываете документ через надежную и проверенную компанию. : kingcityrp.moibb.ru/viewtopic.php?f=34&t=1053
Lazrzrf
| #
Диплом ВУЗа России!
Без наличия диплома очень непросто было продвигаться вверх по карьере. Именно поэтому решение о покупке диплома стоит считать рациональным. Заказать диплом института medforum.5nx.ru/posting.php?mode=post&f=4
Pingrutty
| #
darkmarket url https://github.com/aresonioncq0a7/aresonion – darknet sites
Kxyufaita
| #
darkmarkets darknet market lists
Sazrxex
| #
Мы изготавливаем дипломы любых профессий по приятным ценам.– diplom-top.ru/kupit-diplom-s-zaneseniem-v-reestr-bistro-i-nadezhno-6/
Billydrula
| #
elonbet casino
arhipkacaree
| #
Экскурсии в Геленджике и Архипо-Осиповке: контакты, жмякай здесь
shashlikyug
| #
заказать курицу табака https://shashlikyug.ru
MarkNOlaf
| #
darknet markets bitcoin dark web
Rabyitabe
| #
dark web market https://github.com/darkwebsitesyhshv/darkwebsites – dark web market urls
Steel_Nomad
| #
Мне самому помог этот ресурс https://anex-chel.ru по финансам.
Nikigboith
| #
На сайте https://antipoligraf.ru/ почитайте про Институт прикладной психофизиологии. Здесь ведется профессиональная, комплексная подготовка к тому, чтобы пройти полиграф. Учат и тому, чтобы быстро, безопасно и комплексно снять стресс при помощи уникальных и инновационных приборов обратной связи. И самое важное, что все это абсолютно безопасно, при этом гарантированно поможет. Кроме того, ведется обучение саморегуляции при помощи обратной связи. У института огромное количество партнеров, которые стараются работать в комплексе.
DonDonfaita
| #
darkmarket list https://github.com/nexusdarkrtv1u/nexusdark – dark web markets
Vincenttef
| #
автоматизация бизнес-процессов
CyberWalker
| #
Успешные кейсы применения финансов: https://kotofey-nsk.ru
WilliamIRrutty
| #
dark market 2025 darknet site
mostbet_gqEn
| #
мостбет скачать https://mostbet6005.ru .
DannyTox
| #
Большой выбор из 8677 наименований на складе. В розницу по оптовые ценам от производителя. Доступная доставка в Москву курьерской службой до 2х дней. Фабричное производство: купить сигареты в москве
Victordiesy
| #
Ванга: правда, мифы и 5 самых известных пророчеств
https://x.com/NargisEhme94100/status/1905737028656136280
WilsonUnine
| #
овоши на мангале купить кебаб
Duanefance
| #
Топ сайтов кейсов CS2 http://ggdrop.cs2-case.org проверенные сервисы с высоким шансом дропа, промокодами и моментальными выводами. Только актуальные и безопасные платформы!
mostbet_jwEn
| #
мостбет промокод https://www.mostbet6005.ru .
Steel_Guardian
| #
Шутки про финансы: https://sber-finans.ru
FNDaviditabe
| #
dark market list darknet markets onion address
Kxyufaita
| #
dark market list darkmarkets
Hunterbax
| #
Землетрясение в Бангкоке: что стало причиной?
https://x.com/SebiBilalova/status/1905743685712855518
DavidBoash
| #
купить анализы гепатиты купить справку анализы
1win_vmOi
| #
1win онлайн 1win онлайн .
Volodyanaf
| #
onion dark website darkmarket link
MarkNOlaf
| #
darkmarkets darkmarket link
AndreDap
| #
Букет шикарный! Все цветы как живые.
букет пионов
1win_auSr
| #
1.вин https://www.1win6019.ru .
KennethHew
| #
Энергосберегающие стеклопакеты для утепления дома — смотрите детали тут https://say.la/Oknavspb
Vincenttef
| #
https://intekey.ru/
zaimpodptskemerovosig
| #
кредит под залог машины
avtolombard-pid-zalog-pts.ru/kemerovo.html
займ птс
mostbet_xcoa
| #
mostbet chrono hiend.borda.ru/?1-16-0-00000259-000-0-0-1743052953 .
mostbet_jyer
| #
мостбет скачать на андроид https://alfatraders.borda.ru/?1-0-0-00004917-000-0-0-1743053068 .
1win_vrOi
| #
wan win wan win .
mostbet_owEn
| #
поддержка мостбет https://www.mostbet6005.ru .
Vincenttef
| #
Создание QR кодов
1win_swSr
| #
что такое 1win http://1win6019.ru/ .
Byte8399
| #
Лучшие материалы по финансам: https://galantdzr.ru.
mostbet_gboa
| #
скачать мостбет https://www.hiend.borda.ru/?1-16-0-00000259-000-0-0-1743052953 .
mostbet_jser
| #
мост бет https://alfatraders.borda.ru/?1-0-0-00004917-000-0-0-1743053068/ .
Diplomi_wzka
| #
продам диплом о высшем образовании продам диплом о высшем образовании .
VortexV2
| #
Пошаговая инструкция по финансам: https://persona-pk.ru
WilliamIRrutty
| #
dark market url darknet markets url
Vincenttef
| #
Создание QR кодов
1win_gfOi
| #
1вин официальный сайт http://obmen.forum24.ru/?1-1-0-00004428-000-0-0-1742816292/ .
1win_xhSr
| #
1win официальный сайт регистрация http://www.1win6019.ru .
FNDaviditabe
| #
dark web market links dark market url
Kxyufaita
| #
dark web link darknet markets 2025
mostbet_vjoa
| #
мастбет http://hiend.borda.ru/?1-16-0-00000259-000-0-0-1743052953 .
mostbet_jjer
| #
mostbest mostbest .
remcaree
| #
Ремонт электронники в Москве – сервисный центр, который может отремонтировать все ссылка
MarkNOlaf
| #
darknet markets onion darknet links
asgdh_fiche
| #
Engaged in fraud
PhantomZ6
| #
Как избежать проблем с финансами? https://sarma-trucks.ru
Vincenttef
| #
Создание QR кодов
Thunder_Hunter
| #
Мне самому помог этот ресурс по финансам: https://kamsf.ru
WilliamIRrutty
| #
darkmarket 2025 tor drug market
Vincenttef
| #
автоматизация бизнес-процессов
HevimeBuike
| #
Ищете топливные карты для юридических лиц и ИП? Обратите внимание на единую топливную карта ЛИОТЭК для бизнеса. Узнайте на сайте https://liotec.ru/ все преимущества выгодной карты Лиотек и какие огромные преимущества она дает, по сравнению с другими картами. Подробнее на сайте.
Volodyanaf
| #
darknet site dark web link
Vincenttef
| #
Создание QR кодов
Vincenttef
| #
автоматизация бизнес-процессов
FNDaviditabe
| #
dark web marketplaces bitcoin dark web
Kxyufaita
| #
dark web marketplaces dark markets 2025
MarkNOlaf
| #
darknet market links darkmarket url
Rohawrdtop
| #
Visit https://gorillacasino-best.com/ we have created a fun, informative and engaging online space where casino lovers, newbies and industry experts can find honest insights, in-depth analysis and valuable strategies in the world of online gambling. Unlike many casino blogs, we are not owned or operated by any gambling company. We provide unbiased content.
zaimpodptsmsksig
| #
ломбард деньги под залог птс
avtolombard-pid-zalog-pts.ru
кредит под залог птс москва
Vincenttef
| #
Glory Casino app
BlueV3
| #
Видел прогнозы, что Финансы будет главным https://finkredit38.ru трендом. Соглашусь?
наука инновации
| #
Инновационные Технологии — это платформа, где
собираются люди, интересующиеся новыми технологиями
и инновациями. Здесь вы найдете новости, статьи,
обзоры и анализ последних достижений
в области науки, техники и промышленности.
Тематика сайта охватывает
новости в – центр инновационных технологии искусственный интеллект,
робототехнику, интернет вещей,
биотехнологии, нанотехнологии, космос, энергетику
и многое другое. Кроме того, авторы и эксперты могут публиковать статьи и делиться своим опытом и
знаниями.
LabocnLef
| #
Аренда комфортабельных катеров и яхт в Санкт-Петербурге от https://7futov.spb.ru/ это удобная возможность осуществить водные прогулки по СПб. У нас большой выбор маршрутов и организация мероприятий под ключ. Самые выгодные цены для аренды и большой выбор прогулочных катеров хоть для одного, хоть для большой компании. Посмотрите все наши катера и яхты на сайте по выгодным ценам!
WilliamIRrutty
| #
darkmarket link dark web market urls
Roberttax
| #
В Волна Казино регулярно проводятся розыгрыши, акции и турниры с щедрыми наградами: казино волна официальный сайт
Kxyufaita
| #
dark websites dark web market list
MarkNOlaf
| #
darknet markets onion darknet site
Storm639
| #
Типичные промахи в финансах: https://zalogvl.ru
Roberttax
| #
Присоединяйтесь к Волна Казино и получайте бонусы не только за регистрацию, но и за активную игру https://volna7777.casino/
Volodyanaf
| #
dark web market urls dark web link
WilliamIRrutty
| #
dark market link best darknet markets
FNDaviditabe
| #
dark web market links darknet drug store
zaimpodptsnovosibsig
| #
деньги в кредит под залог машины
avtolombard-pid-zalog-pts.ru/nsk.html
деньги под залог птс круглосуточно
MarkNOlaf
| #
dark web market list darkmarket 2025
Night93
| #
Ответы на частые вопросы о финансах: https://saturn03.ru
WilliamIRrutty
| #
darknet market darknet drug market
lepigevKit
| #
Компания СанОбработка специализируется на предоставлении компетентных услуг по дезинсекции, дезинфекции, дератизации. Даем гарантию на высочайший уровень безопасности и действенности. Обращайтесь к нам тогда, когда вам надо. https://sanobrabotkarf.ru – тут можете с отзывами клиентов ознакомиться. Наши компетентные специалисты готовы к вам на помощь приехать в любое время. Они проведут тщательную обработку и будут использовать сертифицированные европейские препараты. Проблема решится, ваше жилище станет безопасным и комфортным.
Vincenttef
| #
Glory Casino app
Kxyufaita
| #
darknet markets 2025 darknet sites
Rafaelpluch
| #
телеграмм канал hr хантеров В современном мире HR-специалисты сталкиваются с необходимостью оперативной коммуникации и обмена опытом. HR чат становится незаменимым инструментом для решения этих задач. Разнообразие платформ предоставляет широкие возможности для HR-профессионалов: от специализированных чат-ботов до телеграм-каналов.
MarkNOlaf
| #
dark market link darknet markets onion address
Volodyanaf
| #
darknet markets best darknet markets
1win_rhml
| #
how to bet on 1win 1win12.com.ng .
DragonNomad
| #
ТОП-10 https://udarnik35.ru ресурсов по финансам.
WilliamIRrutty
| #
darknet marketplace dark market
Vincenttef
| #
бесплатный онлайн генератор QR кодов
Vincenttef
| #
Лучший генератор QR-кодов
Kxyufaita
| #
darknet websites darkmarket url
Derricksaisy
| #
Заказала цветы для второй половинки – любовь
доставка цветов
MarkNOlaf
| #
darknet markets links darkmarket list
Vincenttef
| #
бесплатный онлайн генератор QR кодов
MetaMask Download
| #
MetaMask Download was the best decision I made. Now I can access my assets anytime, anywhere, with full security.
zaimpodzalogptsekbsig
| #
автоломбард залог птс
zaim-pod-zalog-pts1.ru/ekb.html
деньги под залог птс екатеринбург круглосуточно
Trefstq
| #
Приобрести документ о получении высшего образования можно у нас. diplomd-magazinp.ru/kupit-attestat-za-9-klass-4-5
WilliamIRrutty
| #
darkmarket link dark web market urls
Tolodtsnozy
| #
На сайте https://lilibum.ru вы обязательно найдете вторую половинку для того, чтобы пообщаться, завести знакомства, найти человека для любви, серьезных отношений. Здесь ни одна сотня анкет, среди которых вы точно найдете того, кто симпатичен. Сразу напишите ему и пригласите на долгожданное свидание, чтобы пообщаться, узнать о его интересах, а потом, возможно, и создать семью. Постоянно выкладываются новые анкеты для того, чтобы вы обязательно подобрали родственную душу для любых целей. Для доступа ко всем функциям пройдите регистрацию.
Vincenttef
| #
Создание QR кодов
FNDaviditabe
| #
darknet markets 2025 darknet markets onion
Volodyanaf
| #
dark web market list dark web marketplaces
mostbet_weEl
| #
motsbet https://www.severussnape.borda.ru/?1-10-0-00000023-000-0-0-1743053372 .
Billydrula
| #
elonbet casino
MarkNOlaf
| #
darkmarket dark web market urls
mostbet_ajEl
| #
mostbet kg отзывы mostbet kg отзывы .
mostbet_ueOr
| #
mostbet kg отзывы mostbet kg отзывы .
1win_qnOt
| #
партнёрка 1win http://fanfiction.borda.ru/?1-0-0-00029708-000-0-0-1743051664/ .
panupFride
| #
Сервис бесплатных объявлений №1 хорошо зарекомендовал себя, о чем свидетельствуют отзывы. Плюсы: удобный интерфейс, огромная база объявлений, широкий выбор категорий для размещения и поиска предложений. Заходите и регистрируйтесь. Найдите своего покупателя! Ищете транспорт доска бесплатных объявлений? Ynla.ru – тут с мошенниками меньше вероятности столкнуться. Несмотря на скромный интерфейс, сайт достаточно функционален. Регистрация занимает всего несколько минут. Сервис наш для ПК, планшетов и смартфонов оптимизирован. Настоящей доской объявлений пользуйтесь!
Vincenttef
| #
Лучший генератор QR-кодов
mostbet_xxOr
| #
mostbet kg скачать на андроид cah.forum24.ru/?1-3-0-00000096-000-0-0-1743053764 .
1win_bqOt
| #
1win партнерка вход https://www.fanfiction.borda.ru/?1-0-0-00029708-000-0-0-1743051664 .
WilliamIRrutty
| #
darknet sites darknet sites
mostbet_mdMn
| #
мостбет скачать бесплатно https://assa0.myqip.ru/?1-23-0-00000149-000-0-0-1743053201/ .
mostbet_xhEl
| #
мостбет официальный сайт мостбет официальный сайт .
mostbet_scMn
| #
мостбет промокод http://assa0.myqip.ru/?1-23-0-00000149-000-0-0-1743053201/ .
mostbet_smOr
| #
mostbet скачать на телефон бесплатно андроид cah.forum24.ru/?1-3-0-00000096-000-0-0-1743053764 .
mostbet_dpMn
| #
мосбет assa0.myqip.ru/?1-23-0-00000149-000-0-0-1743053201 .
1win_nxOt
| #
1 вин. https://fanfiction.borda.ru/?1-0-0-00029708-000-0-0-1743051664/ .
1win_lwml
| #
1win sports http://www.1win12.com.ng .
AntonioHaush
| #
Nucleus Earn is revolutionizing DeFi staking and passive income generation by offering secure, high-yield crypto rewards. With smart contract-powered staking pools, Nucleus Earn allows users to earn rewards effortlessly while maintaining full control over their assets. Whether you’re a beginner or an experienced investor, Nucleus Earn’s decentralized staking platform ensures transparency, security, and optimal returns in the fast-growing world of DeFi. https://nucleusearn.org
Vincenttef
| #
https://qrkoder.ru/
Billydrula
| #
elonbet casino BD
KennethHew
| #
Рассрочка и кредит на окна без переплат — подробнее вот здесь https://secure.smore.com/n/8ex15-okna-v-spb-ru
KennyHut
| #
UnagiSwap is a cutting-edge decentralized exchange (DEX) that provides fast, secure, and transparent crypto trading. Designed for traders looking to swap digital assets efficiently without intermediaries, UnagiSwap offers low fees, deep liquidity, and seamless smart contract execution. Whether you’re a casual trader or a professional investor, UnagiSwap’s non-custodial platform ensures full control over your assets in a decentralized environment. https://unagiswap.org
Gregorybek
| #
Perena is a cutting-edge blockchain development platform designed to empower decentralized applications (dApps) with scalable and secure solutions. Built for Web3 innovators, Perena blockchain solutions offer seamless integration, high-performance smart contracts, and decentralized infrastructure. Whether you’re building DeFi protocols, NFT marketplaces, or enterprise blockchain applications, Perena provides the tools needed to scale with confidence. https://perena.tech
giyujhouby
| #
ВудХаус495 – компания, которая квалифицированные услуги предоставляет. Мы реализовали больше 20 000 строительно-отделочных работ деревянных домов. Гарантируем лучшую цену на рынке. Договор составляем, в котором все моменты детально прописываем. http://woodhouse495.ru – здесь представлена более детальная информация о нас. Используем только качественные материалы. В кратчайшие сроки выполняем поставленные перед нами задачи. Предоставляем каждому клиенту индивидуальный подход. Заполните форму на сайте, и мы перезвоним вам в ближайшее время.
Robertskits
| #
Upshift Finance is a next-generation decentralized trading platform designed to provide secure, fast, and efficient crypto transactions. With smart contract automation, low transaction fees, and seamless integration with DeFi protocols, Upshift Finance empowers traders to swap digital assets and execute trades with maximum security. Whether you’re a beginner or an experienced trader, Upshift Finance offers a powerful, transparent, and user-friendly trading ecosystem. https://upshift.ink
Vincenttef
| #
автоматизация бизнес-процессов
prodvijenie saita_myOr
| #
раскрутка сайта в топ http://www.puzzleweb.ru/recl3/effjektivnoje-prodvizhjenije-sajtov-v-moskvje-kak-dostich-vysokikh-rjezultatov.php/ .
Full Report
| #
Full Report
blog topic
xejonbed
| #
Типография Copy General окажет помощь в воплощении ваших идей в реальность. Мы понимаем то, что каждый наш проект уникальным является и особого подхода требует. Ценим время клиентов. Гарантируем высочайшую скорость осуществления заказов. https://copygeneral.ru – тут с отзывами заказчиков можете ознакомиться. Copy General – типография, на которую вы спокойно можете положиться. Постоянно над новыми решениями работаем, чтобы помогать своим клиентам задачи решать с дизайном, маркетингом и полиграфией. Применяйте преимущества нашей типографии!
Vincenttef
| #
Лучший генератор QR-кодов
FevemtJem
| #
На сайте https://lufkad.com/ вы сможете заказать качественные, сертифицированные и оригинальные мансардные окна премиального качества и по привлекательной стоимости. Конструкции LUFKAD созданы по уникальным и инновационным технологиям, потому отличаются безупречным качеством, долгим сроком эксплуатации. LUFKAD считается такой компанией, которая выполняет все необходимые работы и в полном объеме. Многие производственные технологии, которые применяются этой компанией, не имеют аналогов. Заказывайте окна по лучшей стоимости.
1win_ivml
| #
1win sports betting https://1win12.com.ng .
KiriyvoLauff
| #
Сайт https://t.me/azino777_a является официальным каналом популярного онлайн-заведения «Azino 777». Только здесь находятся самые последние новости, а также увлекательные анонсы слотов, турниров. На сайте также публикуются и бонусы, а также промокоды, которые позволят сэкономить. Заходите на этот сайт для того, чтобы получить больше полезной, важной, содержательной информации на данную тему. С этого канала вы сможете сразу перейти на официальный сайт. Теперь все новости из сферы игр находятся в вашем мобильном.
prodvijenie saita_keOr
| #
раскрутка сайта в москве http://www.puzzleweb.ru/recl3/effjektivnoje-prodvizhjenije-sajtov-v-moskvje-kak-dostich-vysokikh-rjezultatov.php .
Vincenttef
| #
автоматизация бизнес-процессов
RobertDaw
| #
Non Gamstop casinos offer the best variety—super cool!
non gamstop onlnie casino lightning roulette
More Info
| #
More Info
blog topic
1win_gfOt
| #
1хwin http://admiralshow.forum24.ru/?1-17-0-00000242-000-0-0-1742816555/ .
1win_zzOt
| #
1хwin admiralshow.forum24.ru/?1-17-0-00000242-000-0-0-1742816555 .
Vincenttef
| #
https://orgnaztech.mirtesen.ru/blog/43202871346/Gde-Kazhdyiy-Gost-Osobennyiy-Otel-Glory
MelvinROF
| #
Лучшие сайты кейсов ggdrop.casecs2.com в CS2 – честный дроп, редкие скины и гарантии прозрачности. Сравниваем платформы, бонусы и шансы. Заходи и забирай топовые скины!
1win_wzOt
| #
1 вин https://admiralshow.forum24.ru/?1-17-0-00000242-000-0-0-1742816555/ .
prodvijenie saita_cqOr
| #
сайтов продвижение http://www.puzzleweb.ru/recl3/effjektivnoje-prodvizhjenije-sajtov-v-moskvje-kak-dostich-vysokikh-rjezultatov.php/ .
Cazrtir
| #
Купить диплом о высшем образовании!
Мы можем предложить документы университетов, расположенных в любом регионе РФ. Документы выпускаются на бумаге высшего качества: opengroup.net/include/pgs/kupit_diplom_o_srednem_obrazovanii_29.html
RobertDaw
| #
Best non Gamstop casino for roulette is unreal—nice!
non gamstop no deposit casino
1win_zgPi
| #
1 win официальный http://1win6020.ru/ .
Vincenttef
| #
https://intekey.ru/
zaimpodptskazancoN
| #
автоломбард в казани под залог
avtolombard-pid-zalog-pts.ru/kazan.html
автоломбард
Vincenttef
| #
https://intekey.ru/
RobertDaw
| #
Non-Gamstop casinos are the real deal—highly recommend!
new non gamstop casinos no deposit bonus
Dnrtcga
| #
Где купить диплом по нужной специальности?
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам. Мы готовы предложить документы техникумов, которые расположены на территории всей Российской Федерации. Можно заказать диплом за любой год, включая документы старого образца. Документы печатаются на “правильной” бумаге самого высшего качества. Это позволяет делать настоящие дипломы, не отличимые от оригиналов. Документы заверяются необходимыми печатями и подписями. Всегда стараемся поддерживать для заказчиков адекватную политику тарифов. Важно, чтобы документы были доступны для подавляющей массы наших граждан. diplom-zakaz.ru/kupit-diplom-vuza-6
1win_gbsi
| #
1вин войти http://www.realistzoosafety.forum24.ru/?1-11-0-00001540-000-0-0-1742816894 .
1win_ymMa
| #
игра 1вин https://naigle.borda.ru/?1-17-0-00000329-000-0-0-1742816734 .
Vincenttef
| #
https://intekey.ru/
1win_risi
| #
1win kg скачать realistzoosafety.forum24.ru/?1-11-0-00001540-000-0-0-1742816894 .
1win_uiMa
| #
one win https://naigle.borda.ru/?1-17-0-00000329-000-0-0-1742816734 .
1win_bjPi
| #
1вин кыргызстан http://1win6020.ru .
1win_xpsi
| #
1win казино https://www.realistzoosafety.forum24.ru/?1-11-0-00001540-000-0-0-1742816894 .
1win_roMa
| #
1vin kg https://naigle.borda.ru/?1-17-0-00000329-000-0-0-1742816734 .
Arthurver
| #
Рэпер Паша Техник находится в критическом состоянии в Таиланде
https://x.com/NargisEhme94100/status/1906094610788573414
Vincenttef
| #
https://qrkoder.ru/
Kxyufaita
| #
dark web market links darknet market lists
FNDaviditabe
| #
dark market darknet drug market
Zadatelwrino
| #
На сайте https://t.me/official_izzicasino/ представлены новости, которые будут вам интересны. Так, находясь в любом месте, вы получите возможность отслеживать появление новостей, свежих данных, полезной информации, которая вам обязательно пригодится. Здесь всегда публикуются промокоды, акции, которые сделают игру более интересной, увлекательной, динамичной. Важная информация выкладывается здесь регулярно, чтобы вы были в курсе последних событий. Теперь вся ценная информация окажется в одном месте.
RobertDaw
| #
Non Gamstop casinos no deposit offers are perfect for testing games—cool!
no gamstop casino
Diplomi_auKt
| #
Купить диплом университета!
Мы готовы предложить документы университетов, расположенных на территории всей России.
diplomt-v-chelyabinske.ru/kupit-attestat-za-9-klass-v-moskve-2
MarkNOlaf
| #
dark web link darkmarket link
Vincenttef
| #
автоматизация бизнес-процессов
1win_hcPi
| #
1win live 1win6020.ru .
zaimpodptskemerovosig
| #
деньги под залог авто
zaim-pod-zalog-pts1.ru/kemerovo.html
займ денег под залог автомобиля
PhillipHit
| #
Video surveillance software plays a crucial role in both residential and commercial security. Security camera software and security surveillance software provide real-time video monitoring and recording. IP camera software for PC, IP camera recording software, and network video recorder software enable secure video storage. VMS software, such as VMS CCTV software and VMS security camera software, supports large-scale surveillance operations. AI video surveillance software and artificial intelligence CCTV software improve security by detecting objects and movements automatically. Cloud video surveillance software and remote video surveillance software offer flexible access to surveillance footage. Motion detection software enhances security by alerting users to unusual activity. smart vision ip camera software
Volodyanaf
| #
darkmarket url dark web market
WilliamIRrutty
| #
dark web drug marketplace darknet markets url
Sazrrdv
| #
Мы предлагаем дипломы любой профессии по приятным ценам. О преимуществах заказа документов в нашей компании
Вы приобретаете документ в надежной и проверенной временем компании. Это решение сэкономит не только средства, но и ваше драгоценное время.
На этом плюсы не заканчиваются, их куда больше:
• Дипломы делаем на подлинных бланках с мокрыми печатями и подписями;
• Дипломы всех высших учебных заведений РФ;
• Цена намного ниже той, которую потребовалось бы платить на очном обучении в университете;
• Максимально быстрая доставка в любые регионы Российской Федерации.
Заказать диплом о высшем образовании– http://soocian.com/read-blog/6145_diplom-ob-obrazovanii.html/ – soocian.com/read-blog/6145_diplom-ob-obrazovanii.html
Vincenttef
| #
https://intekey.ru/
RobertDaw
| #
Just signed up at a non Gamstop casino—loving the variety so far!
non gamstop casino 2021
Vincenttef
| #
Создание QR кодов
gemavliers
| #
UpakovkaRus – компания, которая характеризуется надежностью и выгодной ценой. Производим приличный выбор изделий из ПВХ и спанбонда. Используем материалы, отличающиеся высочайшим качеством. Всегда любые пожелания и требования заказчиков учитываем. https://upakovkarus.ru – здесь представлены примеры готовой продукции. Если не отыщите необходимый вариант, пришлите нам фото вашей модели, такую же осуществим. Вы можете связаться с нами в любое время, контакты на ресурсе указаны. Нашими клиентами мы дорожим. Ждем от вас заявок!
Ап-Х официальный сайт
| #
Добро пожаловать в Up X Casino, где ваши мечты об азартных играх станут реальностью. Здесь представлены широкий ассортимент азартных игр, включая видеослоты, карточные игры и рулетку. И это еще не все! Для наших игроков доступны регулярные турниры и акции, которые помогают увеличить шансы на успех. По данным статистики, активные игроки достигают лучших результатов. Когда стоит испытать удачу? Ответ прост: начните прямо сейчас! https://russkij-tekst.ru/. Какие шаги предпринять перед игрой? Изучите правила и условия использования платформы для успешного начала вашей игровой сессии. Используйте специальные условия для постоянных игроков, чтобы получить максимум удовольствия и выигрыша. Попробуйте бесплатные слоты, чтобы освежить навыки, чтобы вернуться в игровой ритм без риска.
RobertDaw
| #
Non Gamstop UK casino has a slick design—totally hooked!
new non gamstop casino
Vincenttef
| #
https://intekey.ru/
zaimpodptsmsksig
| #
автоломбард под залог
zaim-pod-zalog-pts1.ru
кредит под залог птс авто
GetufGek
| #
Наша компания https://etbpro.ru/ предлагает комплексные решения для проектирования, монтажа, модернизации и технического обслуживания высокотехнологичных электронных систем: видеонаблюдение, пожарная сигнализация, скуд, электрооборудование, охранная сигнализация и автоматизированные системы управления. ООО ЭТБ Ваш надежный партнер в обеспечении безопасности. Подробнее на сайте.
Vincenttef
| #
Создание QR кодов
KosemtMug
| #
На сайте https://mybeterex.com/ вы сможете уточнить всю необходимую информацию по поводу надежной и проверенной компании «BETEREX», которая предлагает полный комплекс услуг, связанных с ведением бизнеса в Китае. У вас появляется возможность заказать доставку грузов, товаров. Кроме того, вы сможете рассчитывать на получение всех необходимых образцов, а также переговоры с другой стороной. Будут решены все вопросы, независимо от сложности. Воспользуйтесь профессиональной консультацией, чтобы уточнить информацию.
1win_pcml
| #
1win home 1win12.com.ng .
RobertDaw
| #
Non Gamstop casinos no deposit offers are too good to miss—great!
casino no on gamstop
Vincenttef
| #
Создание QR кодов
1win_mvsl
| #
1win сайт вход http://1win6001.ru .
KefuspBiC
| #
Ищете куда сходить в Ижевске? Посетите сайт https://izhfun.ru/ и вы найдете главные развлечения Ижевска и где отдохнуть. Выбирайте категории: кафе и рестораны, квизы и игры, театры, концерты, другие мероприятия и места и смотрите текущие или будущие события. Выбирайте интересное времяпрепровождение вместе с нами! Самая актуальная информация на нашем сайте как получить незабываемые эмоции!
buy iptv
| #
buy iptv
A Brief History of Elemental Analysis » Artemis Analytical
IPTV Box Store
| #
IPTV Box Store
A Brief History of Elemental Analysis » Artemis Analytical
Vincenttef
| #
Создание QR кодов
RobertDaw
| #
Non Gamstop casinos no deposit deals keep me coming back—great!
non gamstop casinos netent
zaimpodptsnovosibsig
| #
кредит под птс грузового автомобиля
zaim-pod-zalog-pts1.ru/nsk.html
кредит залог птс в новосибирске
PhillipHit
| #
This resource specializes in video surveillance software, featuring reviews of security camera software for Windows, IP camera software for PC, and surveillance software for PC. It includes in-depth evaluations of IP camera recording software and motion detection software, ensuring users have access to the latest video security software. The platform also covers cloud video surveillance software and VMS software, helping users integrate these tools into their overall security systems. Whether looking for surveillance software for PC or advanced IP camera solutions, this site provides essential guidance. smartvision best video surveillance software
Vincenttef
| #
https://orgnaztech.mirtesen.ru/blog/43202871346/Gde-Kazhdyiy-Gost-Osobennyiy-Otel-Glory
1win_jqml
| #
1win online https://www.1win12.com.ng .
EdwardBiorn
| #
Цвет не выгорает на солнце!
женский костюм купить томск
RobertDaw
| #
Non-Gamstop casinos offer the best escape—highly recommend!
non gamstop no deposit casino
1win_ugsl
| #
1win ставки официальный сайт https://1win6001.ru .
Vincenttef
| #
Лучший генератор QR-кодов
prodvijenie saita_wmOr
| #
seo продвижение сайтов seo продвижение сайтов .
Kxyufaita
| #
tor drug market onion dark website
FNDaviditabe
| #
dark web market darkmarket list
Vincenttef
| #
Glory Casino app
mostbet_piei
| #
mostbet kg скачать на андроид http://mostbet6006.ru .
1win_vemr
| #
1-win https://www.familyclub.borda.ru/?1-6-0-00002163-000-0-0-1743051813 .
1win_tcPi
| #
1win.pro 1win6020.ru .
MarkNOlaf
| #
dark market darknet markets
zaimpodzalogptsekbsig
| #
оформить кредит под залог авто
avtolombard-11.ru/ekb.html
деньги под залог машины екатеринбург
pubuwbuh
| #
АнтикорМастер предлагает квалифицированные услуги. Наша политика ценообразования прозрачна. Стараемся осуществить процесс ухода за вашим авто легким и долгосрочным. Запишитесь на бесплатную диагностику, чтобы выяснить состояние вашего антикоррозийного покрытия. https://xn—-7sbbummpeluekfi.xn--p1ai/ – здесь узнаете, почему нас выбирают. Предлагаем чистку лазерную, которая не оставляя следов, эффективно ржавчину убирает. Предоставляем до 10 лет на осуществленные работы письменную гарантию. Оставьте личные контакты на сайте, и мы с вами свяжемся.
Jariornsw
| #
Где заказать диплом по нужной специальности?
Наши специалисты предлагают быстро и выгодно заказать диплом, который выполнен на оригинальном бланке и заверен печатями, водяными знаками, подписями официальных лиц. Диплом способен пройти любые проверки, даже при использовании специально предназначенного оборудования. Достигайте своих целей максимально быстро с нашей компанией.
Приобрести диплом любого ВУЗа diploml-174.ru/kupit-diplom-novogo-obraztsa-14/
Vincenttef
| #
автоматизация бизнес-процессов
SteveNor
| #
https://punkgazetka.forum24.ru/?1-9-0-00002408-000-0-0-1740791014
Sazrhte
| #
Покупка документа о высшем образовании через надежную фирму дарит ряд плюсов. Это решение дает возможность сберечь как личное время, так и серьезные финансовые средства. Впрочем, только на этом выгоды не ограничиваются, достоинств намного больше.Мы готовы предложить дипломы любой профессии. Дипломы производят на настоящих бланках государственного образца. Доступная цена в сравнении с огромными затратами на обучение и проживание в чужом городе. Покупка диплома института является мудрым шагом.
Приобрести диплом о высшем образовании: kupitediplom.ru/diplom-vracha-kupit/
Mazrezn
| #
Где заказать диплом по актуальной специальности?
Мы оказываем услуги по изготовлению и продаже документов об окончании любых ВУЗов РФ. Документы изготавливаются на фирменных бланках. teamswedenclub.com/read-blog/6329_kupit-svidetelstvo-o-razvode.html
Mazrnkd
| #
Где заказать диплом специалиста?
Получаемый диплом со всеми печатями и подписями целиком и полностью отвечает условиям и стандартам Министерства образования и науки Российской Федерации, неотличим от оригинала – даже со специально предназначенным оборудованием. Не следует откладывать свои мечты и цели на продолжительные годы, реализуйте их с нами – отправляйте быструю заявку на изготовление диплома уже сегодня! Купить диплом о высшем образовании – быстро и просто! vacshidiplom.com/kupit-gotovij-attestat/
Sazrzvx
| #
Купить документ университета можно в нашем сервисе. Мы предлагаем документы об окончании любых университетов РФ. Вы получите необходимый диплом по любой специальности, любого года выпуска, включая документы старого образца. Гарантируем, что в случае проверки документа работодателями, подозрений не возникнет. rentry.co/9uc8eqgg
More info
| #
Just wish to say your article is as astonishing.
The clearness to your publish is simply great and i can suppose you’re a professional
on this subject. Fine along with your permission allow me to grab your feed to keep up to date
with approaching post. Thank you a million and please carry on the enjoyable work.
shinomontaj_dmSn
| #
шиномонтаж малаховка шиномонтаж малаховка .
WilliamIRrutty
| #
dark websites dark web drug marketplace
prodvijenie saita_hekn
| #
раскрутка сайтов москва раскрутка сайтов москва .
Kxyufaita
| #
dark websites darknet markets onion address
FNDaviditabe
| #
onion dark website dark web drug marketplace
Vincentduh
| #
Что такое «Порту Пати» в Дубае и почему об этом говорят все
https://x.com/DeyanetKrmv/status/1906296026006220909
Volodyanaf
| #
dark markets 2025 darknet markets onion address
http://gudang.desa.id/pagerank/
| #
http://gudang.desa.id/pagerank/
blog topic
MarkNOlaf
| #
dark market list tor drug market
FivifdvaH
| #
На сайте https://gk-psk72.ru/ закажите звонок для того, чтобы приобрести ЖБИ от производителя. Прямо сейчас воспользуйтесь возможностью изучить всю продукцию, которая обязательно вам пригодится. А для того, чтобы узнать об особенностях работы предприятия, необходимо изучить портфолио. Компания оказывает услуги в строительной деятельности более 10 лет. Всего в каталоге более чем тысяча наименований. Есть возможность воспользоваться комплексным предложением товаров, услуг. Преимуществом обращения в компанию является и небольшая стоимость.
Zokojthboava
| #
На сайте https://t.me/rahvalskiy_team уточните то, какие услуги оказываются популярной и проверенной компанией «RAHVALSKIY TEAM», которая предлагает создать уникальный, функциональный сайт. Он будет работать специально для вас и раскрутки бизнеса. В обязательном порядке разрабатывается особая эффективная стратегия. В компании работают проверенные, знающие и квалифицированные сотрудники, которые учитывают потребности всех клиентов. Воспользуйтесь комплексными услугами для организации бизнеса.
shinomontaj_mtSn
| #
шиномантаж подольск шиномантаж подольск .
WilliamIRrutty
| #
dark web market list darknet markets 2025
1win_dhPi
| #
скачать 1win официальный сайт http://www.1win6020.ru .
prodvijenie saita_jnOr
| #
seo продвижение сайтов seo продвижение сайтов .
1win_jymr
| #
1 win официальный familyclub.borda.ru/?1-6-0-00002163-000-0-0-1743051813 .
Andrewsmick
| #
Say hello to AquaSculpt—a game-changer in weight loss! These AquaSculpt capsules use natural AquaSculpt ingredients to shed pounds and boost confidence. No AquaSculpt side effects, just pure AquaSculpt results—see why in AquaSculpt reviews. Learn AquaSculpt how to use and join thousands who love it. AquaSculpt buy today at http://aquasculpt.best
zaimpodptskazancoN
| #
кредит под залог птс
avtolombard-11.ru/kazan.html
займ под залог авто
Kxyufaita
| #
darkmarkets dark web link
FNDaviditabe
| #
dark web sites bitcoin dark web
prodvijenie saita_gfkn
| #
раскрутка сайтов в москве раскрутка сайтов в москве .
w69 ทางเข้า
| #
w69 ทางเข้า – https://rwho3.com ปรับปรุงอินเทอร์เฟซแอปให้ใช้งานง่ายขึ้น ดีไซน์สวยงาม ค้นหาเกมได้รวดเร็วและสะดวกสบาย
mostbet_vtei
| #
mosbet http://mostbet6006.ru .
Robertwef
| #
Struggling to lose weight? AquaSculpt is transforming weight loss with its natural, fast-acting capsules. Packed with proven AquaSculpt ingredients, these capsules burn fat, boost energy, and deliver real AquaSculpt results in weeks. Curious about AquaSculpt reviews? Users love its effectiveness and zero AquaSculpt side effects. Want to know AquaSculpt how to use? It’s simple—take daily and watch the pounds melt away. Ready to try? AquaSculpt buy now at http://aquasculpt.xyz and sculpt your dream body today!
Brianhig
| #
Say hello to AquaSculpt—a game-changer in weight loss! These AquaSculpt capsules use natural AquaSculpt ingredients to shed pounds and boost confidence. No AquaSculpt side effects, just pure AquaSculpt results—see why in AquaSculpt reviews. Learn AquaSculpt how to use and join thousands who love it. AquaSculpt buy today at http://aquasculpt.one !
MichaelBop
| #
Struggling to lose weight? AquaSculpt is transforming weight loss with its natural, fast-acting capsules. Packed with proven AquaSculpt ingredients, these capsules burn fat, boost energy, and deliver real AquaSculpt results in weeks. Curious about AquaSculpt reviews? Users love its effectiveness and zero AquaSculpt side effects. Want to know AquaSculpt how to use? It’s simple—take daily and watch the pounds melt away. Ready to try? AquaSculpt buy now at http://aquasculpt.me and sculpt your dream body today!
MarkNOlaf
| #
dark web market links darknet market links
shinomontaj_buSn
| #
шиномонтаж щёлково шиномонтаж щёлково .
Arthurbalty
| #
AquaSculpt weight loss is here to stay! With AquaSculpt capsules, you get fast AquaSculpt results thanks to natural AquaSculpt ingredients. No worries about AquaSculpt side effects—users confirm it in AquaSculpt reviews. Curious AquaSculpt how to use? It’s easy and effective. AquaSculpt where to buy? Visit http://aquasculpt.lifestyle and transform your body now!
VogacPeT
| #
На сайте https://doge.coinwatchtoday.ru/ вы узнаете все о криптовалюте Dogecoin (DOGE) – история, новости, торговые идеи, криптобиржи и торговые инструменты, которые позволят вам с выгодой инвестировать или торговать c Dogecoin (DOGE). В отличие от биткоина, DOGE предлагает быстрые и дешевые транзакции, оставаясь при этом удобным средством микроплатежей и благотворительности. Простота и низкие комиссии!
Volodyanaf
| #
darknet market list darknet markets 2025
1win_fymr
| #
1wi https://familyclub.borda.ru/?1-6-0-00002163-000-0-0-1743051813/ .
букмекерские конторы которые дают фрибет
| #
Фрибет: Что Это Такое и Как Его Использовать?
В мире онлайн-ставок и азартных игр термин “фрибет” стал широко известен и популярен среди игроков. Но что это такое, как работает фрибет и как его можно использовать с максимальной выгодой? В этой статье мы разберем все ключевые аспекты, связанные с фрибетами, чтобы вы могли уверенно применять их в своей игре.
Что такое фрибет?
Фрибет (от англ. “free bet” — бесплатная ставка) — это бонус, который https://www.youtube.com/watch?v=eMVTiWJujqQ конторы или онлайн-казино предоставляют своим пользователям. Это своего рода подарок, позволяющий сделать ставку на спортивное событие или сыграть в игру без использования собственных средств. Если ставка выигрывает, игрок получает выигрыш за вычетом суммы самого фрибета, а если проигрывает — не теряет ничего из своего кошелька.
Фрибеты часто используются букмекерами как маркетинговый инструмент для привлечения новых клиентов или поощрения активных пользователей. Это отличный способ попробовать свои силы в ставках, не рискуя личными деньгами.
Виды фрибетов
Фрибеты могут различаться по условиям предоставления и использования. Вот основные типы, с которыми вы можете столкнуться:
Приветственный фрибет
Этот бонус обычно предлагается новым игрокам при регистрации на сайте букмекера. Например, после создания аккаунта и подтверждения личности вы можете получить фрибет на сумму 500 или 1000 рублей.
Фрибет за депозит
Некоторые букмекеры начисляют бесплатную ставку после пополнения счета на определенную сумму. Например, внеся 1000 рублей, вы можете получить фрибет на такую же сумму.
Фрибет за активность
Постоянным игрокам могут начислять фрибеты за регулярные ставки, участие в акциях или выполнение определенных условий, таких как серия ставок на конкретные события.
Безусловный фрибет
Это редкий, но очень желанный вид бонуса, который не требует выполнения сложных условий. Вы просто получаете фрибет и можете использовать его по своему усмотрению.
Фрибет на день рождения
Многие букмекерские конторы радуют своих клиентов небольшими подарками в виде фрибетов в честь дня рождения.
Как получить фрибет?
Процесс получения фрибета обычно прост и занимает немного времени. Вот стандартные шаги:
Регистрация: Создайте аккаунт на сайте букмекерской конторы.
Верификация: Подтвердите свою личность, загрузив необходимые документы (паспорт или другой ID).
Выполнение условий: Ознакомьтесь с правилами акции — это может быть внесение депозита, ввод промокода или ставка на определенное событие.
Активация: В некоторых случаях нужно активировать фрибет в личном кабинете или через поддержку.
После этого фрибет будет зачислен на ваш бонусный счет, и вы сможете использовать его для ставок.
Как использовать фрибет с умом?
Чтобы извлечь максимум пользы из фрибета, важно учитывать несколько моментов:
Изучите условия: У каждого фрибета есть свои правила. Например, минимальный коэффициент для ставки (обычно от 1.5 до 2.0) или ограничение по видам спорта.
Выбирайте событие с высокой вероятностью: Поскольку это бесплатная ставка, стоит выбрать матч или игру, в исходе которых вы уверены.
Не торопитесь: Фрибеты часто имеют срок действия (от 7 до 30 дней), поэтому используйте их обдуманно, а не на первой попавшейся ставке.
Планируйте вывод: Если ставка выиграла, уточните, можно ли сразу вывести выигрыш или нужно выполнить дополнительные условия (например, отыграть сумму).
Плюсы и минусы фрибетов
Плюсы:
Возможность попробовать ставки без риска для кошелька.
Шанс выиграть реальные деньги с минимальными вложениями.
Отличный бонус для новичков, чтобы освоиться на платформе.
Минусы:
Ограничения по использованию (коэффициенты, события, сроки).
Выигрыш часто требует отыгрыша перед выводом.
Не все фрибеты действительно “бесплатны” — иногда нужен депозит.
Популярные букмекеры с фрибетами
На российском рынке многие букмекерские конторы предлагают фрибеты. Вот несколько примеров:
1xСтавка: До 10 000 рублей для новых игроков.
Фонбет: Фрибет за регистрацию и первый депозит.
Леон: Регулярные акции с бесплатными ставками.
Winline: Один из лидеров по щедрым фрибетам без сложных условий.
Перед регистрацией обязательно проверяйте актуальные условия на сайте букмекера, так как предложения часто обновляются.
Советы для новичков
Сравнивайте предложения: Разные конторы дают разные суммы и условия — выбирайте то, что вам подходит.
Не гонитесь за крупными суммами: Иногда маленький фрибет с простыми условиями выгоднее, чем большой с кучей ограничений.
Читайте отзывы: Узнайте, что говорят другие игроки о выводе выигрышей с фрибетов.
Играйте ответственно: Даже с фрибетом не стоит увлекаться сверх меры — азарт должен приносить удовольствие.
kra31cc
| #
Very nice post. I simply stumbled upon your weblog and wished to mention that
I’ve truly loved surfing around your weblog posts. After
all I will be subscribing to your rss feed and I hope you
write once more soon!
WilliamIRrutty
| #
dark web market urls darkmarket list
prodvijenie saita_sgkn
| #
раскрутка сайта москва раскрутка сайта москва .
Kxyufaita
| #
darkmarket url darknet drug store
FNDaviditabe
| #
darknet markets 2025 darknet drugs
MarkNOlaf
| #
dark web market darknet markets 2025
Unlim бесплатные спины
| #
Анлим Казино предлагает игрокам возможность погрузиться в мир азартных игр.
Здесь в наличии множество игровых автоматов,
карточных игр, а также регулярно проводимые турниры,
что дает шанс игрокам увеличить свои шансы на выигрыш и добавляет массу
удовольствия от процесса.
Анлим Казино – это не просто место для игры, но и потрясающий опыт для всех пользователей, независимо от того, играете ли вы с мобильного устройства или с компьютера.
Мы гарантируем постоянное обновление ассортимента игр и организацию увлекательных турнирных состязаний.
В чем преимущество игры в Анлим Казино?
Быстрая регистрация — всего пару шагов, и вы уже готовы начать играть.
Большие бонусы для новичков — мы дарим бонусы на первый
депозит для старта с большими шансами на победу.
Регулярные акции и турниры —
для каждого, кто хочет расширить свои шансы на победу и дополнительные призы.
Круглосуточная поддержка всегда
готова помочь с любыми вопросами о процессе игры.
Богатый выбор игр доступных как на компьютере,
так и на мобильных устройствах.
Присоединяйтесь к нам уже сегодня!
Анлим Казино ждет вас. Готовы к победам? https://unlim-casinomirage.yachts/
Starda казино официальный сайт
| #
Хотите испытать настоящий азарт? Тогда Starda Casino – ваш идеальный выбор!! Здесь собраны разнообразие игровых автоматов от лицензированных провайдеров. https://starda-strike.buzz/ чтобы испытать удачу в топовых слотах!
Что делает Starda Casino лучшим выбором?
Популярные слоты с потрясающей графикой.
Эксклюзивные предложения для постоянных игроков.
Моментальные транзакции в кратчайшие сроки.
Интуитивный интерфейс без лагов и зависаний.
Техподдержка 24/7 готова помочь в любое время.
Испытайте удачу прямо сейчас чтобы получать максимальное удовольствие!
Once Human
| #
web siteniz çok güzel başarılarınızın devamını dilerim. makaleler çok hoş sürekli sitenizi ziyaret edeceğim
zaimpodptskemerovosig
| #
автоломбард в кемерово под залог
avtolombard-11.ru/kemerovo.html
кредит под залог авто
WilliamIRrutty
| #
dark web market urls tor drug market
DavidUrire
| #
Развитие профессиональных качеств Управленческие консультации от Светланы Подкладышевой, серийного предпринимателя с более чем 20-летним опытом. Она обладает уникальной способностью анализировать сложные ситуации и предлагать практические решения, основанные на глубоких знаниях и реальном опыте. Светлана помогает оптимизировать бизнес-процессы, разработать индивидуальные стратегии роста, повысить конкурентоспособность. Например, оптимизация структуры компании, разработка бизнес-моделей, внедрение технологий.
Volodyanaf
| #
dark market darknet markets 2025
официальный сайт даркнет
| #
Great delivery. Great arguments. Keep up the good spirit.
Kxyufaita
| #
dark web market links darkmarket
FNDaviditabe
| #
dark market list dark market list
MarkNOlaf
| #
darknet drug links best darknet markets
Kuxuhruiva
| #
На сайте https://t.me/s/official_izzicasino/ регулярно публикуются свежие и актуальные новости, которые касаются известного виртуального заведения «IZZI Casino». На этом канале вы найдете только содержательную, полезную информацию, которая обязательно пригодится, если являетесь фанатом этого игрового клуба. Здесь регулярно выкладываются анонсы турниров, выигрышные бонусы, а также фриспины и многое другое, что сделает игру более зрелищной, увлекательной. Вся информация об игорном заведении теперь в одном месте.
WilliamIRrutty
| #
dark web sites darknet markets onion
Kxyufaita
| #
dark web marketplaces dark web market
FNDaviditabe
| #
darknet markets dark websites
Volodyanaf
| #
dark market 2025 darkmarket list
XalowwDaype
| #
На сайте https://mos-stroi-alians.ru/ уточните телефон компании, чтобы воспользоваться такой нужной услугой, как кровля крыш независимо от сложности. На предприятии трудятся высококлассные, квалифицированные сотрудники, которые справятся с решением вопроса очень быстро, на профессиональном уровне. В работе применяются только лучшие, инновационные материалы, особые технологии для нужного результата. Работы ведутся и в выходной день. На все услуги даются гарантии, потому как компания уверена в их качестве.
MarkNOlaf
| #
dark market url dark market
AnthonyWifut
| #
Попробуйте https://abfgss53sdbkl33.ru/
1win_obsl
| #
1 win официальный сайт вход 1 win официальный сайт вход .
non-gamstop casino review
| #
non-gamstop casino review
blog topic
WilliamIRrutty
| #
darknet markets links dark web market links
zaimpodptsmsksig
| #
кредит залог птс наличные
avtolombard-11.ru
деньги залог авто москва
AnthonyWifut
| #
Обязательно попробуйте https://abfgss53sdbkl33.ru/
Vozaflmit
| #
На сайте https://sanobrabotkarf.ru/ закажите консультацию для того, чтобы оформить такую полезную услугу, как профессиональная дезинфекция. В компании применяются только проверенные, надежные препараты, которые считаются полностью безопасными для человека, животного. Работы выполняются лучшими сотрудниками, у которых есть сертификаты на оказание услуг, а также строго в оговоренные сроки. Специально для вас качественное, комплексное обслуживание. Сотрудники применяют инновационные, уникальные технологии для достижения результата.
FNDaviditabe
| #
darknet drugs darknet drug market
Volodyanaf
| #
dark web market urls darknet drug market
MarkNOlaf
| #
bitcoin dark web darknet market
Donaldlaf
| #
dark market darknet markets
TugewHaw
| #
Посетите сайт https://pech.pro/ это магазин печей, каминов и все для отделки бани. Зайдите в каталог и вы найдете существенный выбор – камины, печи, котлы отопительные, дымоходы, печи для дачи и многое другое по самым выгодным ценам. Действует доставка по Ярославлю и всей России. Профессионально осуществляем подключение, монтаж отопительных устройств и их защиты от возгорания.
Pingrutty
| #
darknet site darknet markets
Toliknaf
| #
dark markets 2025 darknet marketplace
WilliamIRrutty
| #
darkmarket dark web market links
Rabyitabe
| #
dark market 2025 dark web market urls
Kxyufaita
| #
dark market dark market onion
kra50cc
| #
I’m gone to say to my little brother, that he should also visit this web site on regular basis to get updated from newest reports.
sex hiepdam
| #
I’ve been surfing online more than three hours today, yet I never found
any interesting article like yours. It’s pretty worth enough for me.
In my view, if all web owners and bloggers made good content as you did, the internet will be much more useful than ever before.
DonDonfaita
| #
darknet links darknet markets onion
AnthonyWifut
| #
Обязательно попробуйте https://abfgss53sdbkl33.ru/
MarkNOlaf
| #
darknet sites onion dark website
Volodyanaf
| #
best darknet markets darknet sites
Pingrutty
| #
dark web market dark web sites
AndreDap
| #
Очень доволен! Цветы свежие, доставка быстрая.
букет пионов
zaimpodptsnovosibsig
| #
займ под залог автоломбард
avtolombard-11.ru/nsk.html
автоломбард под залог птс
Donaldlaf
| #
darknet links darknet site
WilliamIRrutty
| #
darkmarket list dark market link
Toliknaf
| #
dark web sites dark market 2025
Kxyufaita
| #
dark markets darkmarket link
Займ с любой кредитной историей - одобрение 99%
| #
Первый займ — бесплатно? Да, это возможно. Мы собрали рейтинг МФО, которые выдают онлайн займ на карту под 0% на 30 дней. Без подвоха, без скрытых комиссий. Деньги доступны любому жителю России всего по паспорту.
Rabyitabe
| #
darknet market links darkmarket 2025
MarkNOlaf
| #
darknet marketplace dark market link
DonDonfaita
| #
bitcoin dark web dark websites
Pingrutty
| #
darknet market links dark market url
WilliamIRrutty
| #
tor drug market darkmarket link
FNDaviditabe
| #
dark markets 2025 dark market link
AnthonyWifut
| #
Советую https://abfgss53sdbkl33.ru/
Donaldlaf
| #
bitcoin dark web best darknet markets
Eanrndu
| #
Для успешного продвижения по карьере понадобится наличие диплома о высшем образовании. Выгодно купить диплом университета у надежной компании: diplom-ryssia.com/kupit-zaregistrirovannij-diplom-bez-lishnix-xlopot/
uşak taksi
| #
Your site is amazing, the articles are great, I will always come to this site and continue to read because you have very nice articles, thank you
1win_ismr
| #
1win вход в личный кабинет http://familyclub.borda.ru/?1-6-0-00002163-000-0-0-1743051813/ .
Toliknaf
| #
dark markets 2025 tor drug market
MarkNOlaf
| #
darknet marketplace darknet market links
Once Human
| #
web siteniz çok güzel başarılarınızın devamını dilerim. makaleler çok hoş sürekli sitenizi ziyaret edeceğim
Nelejdobep
| #
Компания Мега скупка https://mega-skupka.com/ работает по городу Санкт-Петербурге. Нам можно продать ваши старые и новые гаджеты, а именно смартфоны, ноутбуки, iphone, apple, macbook, игровые Пк, Даем на 25% больше других конкурентов на месте.
AnthonyWifut
| #
Попробуйте https://abfgss53sdbkl33.ru/
avtolombardekbsig
| #
займ под залог авто по стс
https://www.bolsadetrabajotafer.com/employer/avtolombard-pid-zalog-pts6393/
деньги под птс автоломбард
Rabyitabe
| #
dark market onion darknet drugs
WilliamIRrutty
| #
dark markets 2025 dark market 2025
Pingrutty
| #
dark market dark market 2025
AnthonyWifut
| #
Советую https://abfgss53sdbkl33.ru/
Kxyufaita
| #
darknet market darknet market
FNDaviditabe
| #
bitcoin dark web darknet sites
Once Human
| #
web siteniz çok güzel başarılarınızın devamını dilerim. makaleler çok hoş sürekli sitenizi ziyaret edeceğim
DonDonfaita
| #
darknet links darknet sites
MarkNOlaf
| #
darkmarkets darknet drug market
site
| #
Thanks for your personal marvelous posting! I really enjoyed reading it, you could be a great
author.I will make sure to bookmark your blog and
will come back very soon. I want to encourage that you continue your
great job, have a nice holiday weekend!
Once Human
| #
web siteniz çok güzel başarılarınızın devamını dilerim. makaleler çok hoş sürekli sitenizi ziyaret edeceğim
Donaldlaf
| #
darknet markets links darknet markets 2025
Ramirojax
| #
кракен ссылка актуальная – кракен маркетплейс, Как использовать Tor для покупок
WilliamIRrutty
| #
darknet sites darknet markets 2025
Toliknaf
| #
best darknet markets darknet markets onion address
Kxyufaita
| #
darknet market lists darkmarket
FNDaviditabe
| #
darkmarkets darknet marketplace
Pingrutty
| #
dark market dark market link
mostbet_hyei
| #
мостбет войти https://mostbet6006.ru/ .
shinomontaj_xqSn
| #
шиномонтаж цены https://hondahybrid.ru/forum/topic/2886-kakie-uslugi-okazyvaet-shinomontazh// .
1win_oesl
| #
1win партнерская программа вход 1win партнерская программа вход .
Rabyitabe
| #
darknet drugs darkmarket link
xitohnam
| #
Fasvek кровельные материалы высокого качества предоставляет. Вся продукция имеет детальное описание с указанием технических параметров и характеристик. Прежде чем заказ сделать, с ними детальнее ознакомьтесь. Цены каждому доступны. Многие люди остались довольны сотрудничеством с нами. https://fasvek.ru – здесь представлены наши работы. Вы узнаете, что дает использование контролита. Сотрудники компании Вектор – настоящие профессионалы своего дела. Если необходима помощь, оставьте на портале ваш контактный номер, и мы в ближайшее время вам перезвоним.
MarkNOlaf
| #
darknet market lists bitcoin dark web
DonDonfaita
| #
darknet market list darknet market lists
avtolombardekbsig
| #
кредит наличными под залог птс
https://www.joboptimizers.com/employer/market9945/
займ залог авто
WilliamIRrutty
| #
dark web sites dark market 2025
LanihDourl
| #
На сайте https://t.me/rahvalskiy_team воспользуйтесь возможностью заказать такую популярную услугу, как продвижение в Нижнем Новгороде и по любому другому городу. В компании работают лучшие, проверенные и талантливые специалисты, которые подберут для вас оптимальный вариант для того, чтобы ваш бизнес стал востребованным. Сотрудники изучат рынок, а также подберут наиболее уместную и работающую стратегию, которая обязательно вам подойдет. Воспользуйтесь комплексными рекламными услугами. Они обойдутся недорого.
Donaldlaf
| #
darknet site darkmarket url
FNDaviditabe
| #
darknet markets url dark web sites
Kxyufaita
| #
darknet sites dark web drug marketplace
Pingrutty
| #
dark web sites dark web marketplaces
Toliknaf
| #
dark market dark web marketplaces
uşak taksi
| #
Your site is amazing, the articles are great, I will always come to this site and continue to read because you have very nice articles, thank you
MarkNOlaf
| #
dark market link darknet marketplace
ZobuxKneex
| #
На сайте https://organ-hall.ru вы сможете получить уникальный опыт, играя в популярное онлайн-заведение «Vodka Casino», которое заполучило огромное количество поклонников благодаря своей честной и прозрачной политике, большому количеству бонусов. Здесь находятся автоматы, промокоды, бонусы для более зрелищной и интересной игры. Этот клуб создан с использованием новых технологий, поэтому предоставляет безупречный уровень сервиса, регулярные выплаты, интересные функции. С этим заведением вы сможете позволить себе больше!
prodvijenie saita_ytkn
| #
продвижение сайтов в москве продвижение сайтов в москве .
Walterheend
| #
Jestem skrajnie niezadowolony witryna Huone. Tresci na niej sa nieaktualne, a pomoc techniczna po prostu nie odpowiada na wiadomosci. Sposob skladania zamowienia to calkowity koszmar: liczne awarie, powolne ladowanie i absolutny brak obslugi. Mam przekonanie, ze administratorzy po prostu lekcewaza uzytkownikow. Nie zuzywajcie swojego czasu i energii i nerwow, sa o wiele godne uwagi opcje!
Rabyitabe
| #
dark web marketplaces dark markets
1win_mhsl
| #
1вин войти https://www.1win6001.ru .
mostbet_yhei
| #
mostbest http://mostbet6006.ru .
AnthonyWifut
| #
Крайне советую https://abfgss53sdbkl33.ru/
fuel pressure sensor
| #
Hi, Neat post. There is a problem with your website in web explorer, might test this?
IE nonetheless is the market chief and a big component to other folks will
miss your magnificent writing because of this
problem.
CecilExign
| #
Посетите интернет-магазин http://sharpsting.ru и оцените разнообразие товаров: от современных гаджетов до полезных аксессуаров. Мы ценим каждого клиента и стремимся предоставить лучший сервис. Присоединяйтесь к числу наших довольных покупателей уже сегодня
WilliamIRrutty
| #
darknet markets links darknet market list
Pingrutty
| #
dark market list darknet market lists
Donaldlaf
| #
dark markets 2025 bitcoin dark web
JosephNum
| #
For depositing in the Aviator betting game(https://ascenddata.co.ke/how-to-deposit-money-in-the-aviator-game-a-step-by-step-guide/), it’s necessary to complete these instructions.
At the beginning, sign into your profile on the website that has Aviator.
Then, open the Wallet tab.
Click on your supported transaction type, such as e-wallet (Neteller, etc.).
Enter the deposit value, make sure all is correct, and finalize the payment.
Most of the time, your gaming funds will be topped up within seconds, allowing you to enjoy the crash game right away!
Keep in mind: fees may vary on the site.
Kxyufaita
| #
bitcoin dark web darknet markets 2025
FNDaviditabe
| #
dark market dark web link
Sazrchd
| #
купить диплом о дополнительном профессиональном образовании
JimmyLothe
| #
http://fabnews.ru/forum/showthread.php?p=92469#post92469
Toliknaf
| #
onion dark website best darknet markets
shinomontaj_vgSn
| #
шиномантаж сергиев посад hondahybrid.ru/forum/topic/2886-kakie-uslugi-okazyvaet-shinomontazh/ .
Rabyitabe
| #
best darknet markets darknet market list
MarkNOlaf
| #
darknet markets links dark web drug marketplace
Volodyanaf
| #
dark web sites darkmarket list
DonDonfaita
| #
darkmarket darknet markets
Pingrutty
| #
darkmarket 2025 darknet site
Derricksaisy
| #
Розы в яркой летней упаковке – страсть и солнце
купить цветы в томске
JimmyLothe
| #
http://gazeta.ekafe.ru/viewtopic.php?f=110&t=38467
WilliamIRrutty
| #
darknet drug store dark web drug marketplace
mostbet_jwei
| #
скачать мостбет http://www.mostbet6006.ru .
Jamesdusty
| #
Цветы свежие, сочные, будто впитали в себя лето!
доставка цветов
GeraldBaf
| #
регистрация дачного дома
Alfonsomuh
| #
Привет, любитель поиграть в контру и получить за это скинов от Гейба. У тебя есть полны и нвентарь и ты ищешь продать скины с выподом на карту . Читай подборку сайтов которые тебе помогут сделать это мгновенно и с удобным способом вывода для тебя.
avtolombardkazancoN
| #
займ под залог авто казань
https://melocasting.com/employer/zaim-pod-zalog-pts-18232/
залог под птс авто
Donaldlaf
| #
dark web market dark web market urls
Kxyufaita
| #
darknet market links onion dark website
FNDaviditabe
| #
darkmarket 2025 darknet market
Ronaldbut
| #
МикоФарм Фунги
Toliknaf
| #
darknet markets links darknet marketplace
taksi uşak
| #
Your site is amazing, the articles are great, I will always come to this site and continue to read because you have very nice articles, thank you
prodvijenie saita_mfkn
| #
продвижение сайтов в москве продвижение сайтов в москве .
MarkNOlaf
| #
dark web sites dark market
Rabyitabe
| #
dark web drug marketplace darkmarket url
Pingrutty
| #
darkmarket url darknet market lists
1win_msmr
| #
1winn https://familyclub.borda.ru/?1-6-0-00002163-000-0-0-1743051813/ .
Once Human
| #
web siteniz çok güzel başarılarınızın devamını dilerim. makaleler çok hoş sürekli sitenizi ziyaret edeceğim
DonDonfaita
| #
darkmarket list darknet market links
Volodyanaf
| #
dark market url dark web drug marketplace
WilliamIRrutty
| #
bitcoin dark web dark market link
Once Human
| #
web siteniz çok güzel başarılarınızın devamını dilerim. makaleler çok hoş sürekli sitenizi ziyaret edeceğim
Donaldlaf
| #
darkmarket darkmarket 2025
Kxyufaita
| #
darknet markets url dark web market list
FNDaviditabe
| #
dark markets 2025 bitcoin dark web
Ronaldbut
| #
https://mycofarm.ru/
Fuyazquini
| #
На сайте https://koch-market.ru/ закажите звонок для того, чтобы воспользоваться услугами детейлинг центра. Есть возможность воспользоваться такими услугами, как оклейка кузова автомобиля, винилография на мотоцикл, тюнинг фар, оклейка антигравийной пленкой. Для того чтобы сориентироваться в выборе, необходимо изучить примеры работ – те проекты, которые реализованы. Также на сайте представлены и содержательные, любопытные статьи на данную тему. Все специалисты, работающие в компании, отличаются большим опытом.
PosoxdBum
| #
На сайте https://t.me/azino777_a вы найдете огромное количество свежих, актуальных и содержательных новостей, которые представлены на тему популярного онлайн-заведения «Azino 777» . Его по достоинству оценило огромное количество посетителей, ведь площадка работает максимально честно, уважает своих посетителей, ценит их время. Именно здесь, в первую очередь, появляются интересные, увлекательные новости. Вы узнаете о них первым. На этом канале публикуются промокоды, бонусы для более зрелищной игры.
kra37at
| #
Undeniably believe that which you said. Your favorite reason seemed
to be on the net the easiest thing to be aware of.
I say to you, I definitely get irked while people consider worries that they
just don’t know about. You managed to hit the nail upon the
top as well as defined out the whole thing without having side effect , people can take a signal.
Will probably be back to get more. Thanks
Toliknaf
| #
dark market onion darknet market lists
AnthonyWifut
| #
Советую https://abfgss53sdbkl33.ru/
Вулкан Платинум слоты с джекпотом
| #
Vulkan Platinum — это не просто казино, а настоящая игровая платформа для тех, кто ценит качество и
надежность. Здесь можно наслаждаться не
только популярными слотами, но и уникальными игровыми предложениями,
которые могут существенно увеличить ваш доход.
Игровой процесс в Vulkan Platinum всегда увлекательный и непредсказуемый.
Что делает Vulkan Platinum платежные методы отличным выбором для игроков?
Мы предлагаем безопасную игровую среду, где каждый момент проходит с максимальным комфортом и без забот.
С нами выигрывать не только приятно, но и выгодно, благодаря нашим бонусам и акциям.
Когда самое время начать игру в Vulkan Platinum?
Чем раньше, тем лучше! Процесс регистрации занимает всего несколько минут, и вы сразу сможете наслаждаться всеми преимуществами казино.
Вот что вас ждет в Vulkan Platinum:
Мы гарантируем безопасные и быстрые выплаты,
а также надежную защиту ваших
данных.
Независимо от того, предпочитаете
ли вы слоты или настольные игры, у нас есть всё
для вашего комфорта.
Для лояльных клиентов Vulkan Platinum всегда подготовит специальные
предложения и бонусы.
Vulkan Platinum — это ваше место
для больших выигрышей и незабываемых моментов. https://vulkan-rush.top/
Pingrutty
| #
darknet sites darknet markets links
Once Human
| #
web siteniz çok güzel başarılarınızın devamını dilerim. makaleler çok hoş sürekli sitenizi ziyaret edeceğim
MarkNOlaf
| #
darknet marketplace dark market onion
Rabyitabe
| #
dark web sites darknet market list
avtolombardkazancoN
| #
автоломбард в казани круглосуточно
http://zenithgrs.com/employer/jabenefits8178/
займ под залог авто
Nivugtelese
| #
Viziteaza site-ul https://betwave.ro/ ?i vei gasi o lista cu toate cazinourile online din Romania, precum ?i recenzii ale acestora. Va prezentam cele mai bune condi?ii de la cazinou cu retrageri rapide. Va spunem ce bonusuri exista pentru reaprovizionare sau inregistrare. Cum sa alegi cel mai bun cazinou online pentru tine – cite?te pe site!
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
DonDonfaita
| #
darknet drug market best darknet markets
WilliamIRrutty
| #
darknet links dark web marketplaces
alex-machine
| #
отзывы ремонт посудомоечных ремонт посудомоечных машин в москве
Ronaldbut
| #
МикоФарм Фунги
uşak taksi
| #
Your site is amazing, the articles are great, I will always come to this site and continue to read because you have very nice articles, thank you
Diplomi_poKt
| #
Купить диплом университета!
Мы можем предложить документы институтов, расположенных в любом регионе Российской Федерации.
diplomk-vo-vladivostoke.ru/kupit-attestat-texnikuma-7
Volodyanaf
| #
bitcoin dark web darknet drugs
Kxyufaita
| #
darkmarket link dark market url
FNDaviditabe
| #
darknet markets onion address dark markets 2025
Donaldlaf
| #
darknet markets 2025 dark market url
Pingrutty
| #
darknet market dark web market
Toliknaf
| #
darknet site darknet drug market
MarkNOlaf
| #
dark web market links darkmarket link
RobertDaw
| #
Casino non Gamstop platforms are perfect for casual gaming—love it!
no gamstop casino
Rabyitabe
| #
dark market url dark markets 2025
1win_wcot
| #
1win партнерка вход http://alfatraders.borda.ru/?1-0-0-00004932-000-0-0-1743258210 .
drenajnie raboti spb_tlst
| #
сделать дренаж на участке цена в спб сделать дренаж на участке цена в спб .
DonDonfaita
| #
dark websites dark market 2025
sofisimo_c_zzen
| #
Откройте для себя новые горизонты на sofisimo.com, увлекательные материалы.
Погрузитесь в уникальный контент sofisimo.com, изучая.
Сайт sofisimo.com – ваша отправная точка, ищите.
sofisimo.com – остров знаний, вас ждут.
sofisimo.com – ваш помощник в обучении, развивая.
Станьте частью сообщества на sofisimo.com, делиться опытом.
sofisimo.com – это источник креативности, необычные решения.
Посетите sofisimo.com для открытия новых возможностей, мир знаний.
sofisimo.com для любителей знаний, где каждый.
sofisimo.com – это больше, чем просто сайт, научатся.
Проведите время с пользой на sofisimo.com, вдохновение.
Каждый день с sofisimo.com – это новая возможность, учиться.
Свяжитесь с сообществом на sofisimo.com, ценятся.
sofisimo.com – ключ к вашему успеху, жаждет.
Узнайте секреты успеха на sofisimo.com, который.
sofisimo.com – это ваша платформа, но это для всех.
sofisimo.com ждет вас, ваши мечты становятся реальностью.
Ищите новую информацию на sofisimo.com, вдохновение не заканчивается.
sofisimo.com: место, где встречаются идеи, где.
comprar muebles https://sofisimo.com/ .
1win_nfMr
| #
вин 1 http://www.1win6002.ru .
drenajnie raboti spb_pdel
| #
дренажно распределительная система дренажно распределительная система .
WilliamIRrutty
| #
dark web drug marketplace dark markets
Ronaldbut
| #
https://mycofarm.ru/
Lazruzg
| #
Приобрести диплом любого ВУЗа!
Мы изготавливаем дипломы любой профессии по приятным тарифам— diplomass.com/ekb-kupit-diplom-4/
mostbet_ldOt
| #
мостюет http://www.mostbet6007.ru .
Jarioryzv
| #
Где приобрести диплом специалиста?
Наши специалисты предлагают быстро заказать диплом, который выполняется на бланке ГОЗНАКа и заверен печатями, водяными знаками, подписями. Данный диплом пройдет любые проверки, даже с применением профессиональных приборов. Решайте свои задачи быстро с нашим сервисом. Заказать диплом о высшем образовании! linux.listbb.ru/viewtopic.php?f=2&t=1301
Mazrgqq
| #
Мы предлагаем дипломы любой профессии по приятным ценам. Стараемся поддерживать для покупателей адекватную ценовую политику. Для нас важно, чтобы дипломы были доступными для большого количества наших граждан.
Покупка диплома, который подтверждает обучение в университете, – это грамотное решение. Заказать диплом университета: diplomoz-197.com/diplom-kupit-ofitsialno-2/
Pingrutty
| #
darknet markets links dark market url
Cazrwwi
| #
Здравствуйте!
Мы готовы предложить дипломы психологов, юристов, экономистов и других профессий по приятным ценам. Стоимость может зависеть от выбранной специальности, года получения и университета: diplomans.com/
Volodyanaf
| #
darknet links darknet markets
Kxyufaita
| #
onion dark website dark market url
FNDaviditabe
| #
dark market best darknet markets
Donaldlaf
| #
dark web sites darknet drug links
DavidUrire
| #
Оптимизация процессов управления Управленческие консультации от Светланы Подкладышевой, серийного предпринимателя с более чем 20-летним опытом. Она обладает уникальной способностью анализировать сложные ситуации и предлагать практические решения, основанные на глубоких знаниях и реальном опыте. Светлана помогает оптимизировать бизнес-процессы, разработать индивидуальные стратегии роста, повысить конкурентоспособность. Например, оптимизация структуры компании, разработка бизнес-моделей, внедрение технологий.
Toliknaf
| #
darknet markets onion address darknet market
avtolombardkemerovosig
| #
автоломбард залог машины
https://jobs.web4y.online/employer/avtolombard-111946/
деньги под залог автомобиля
degiwmup
| #
https://boilers24.com/ and you will find spare parts for boilers of many brands. Here you can buy boxes and control boards, actuators, gas valves, oil nozzles, ignition electrodes, flame sensors and much more. Check out the catalog with the entire range at competitive prices.
Trefkyq
| #
Заказать документ о получении высшего образования вы можете у нас в столице. diplomass.com/kupit-diplom-sssr-2
MarkNOlaf
| #
darknet markets onion dark web sites
https://vodka-fun.top/
| #
Dreaming of big victories? Then welcome to Vodka Casino – the perfect place to play! Here, we offer thousands of slot machines from top studios. https://vodka-fun.top/ and claim generous bonuses!
What exclusive features does our casino offer?
Popular casino games featuring high RTP.
Lucrative promotions for new and existing players.
Guaranteed winnings without delays or hidden fees.
Optimized platform on smartphones and tablets.
Professional service always ready to assist.
Test your luck to enjoy gambling to the fullest!
RobertDaw
| #
Non-Gamstop casino platforms are so intuitive—really nice!
non gamstop casino 2021
Clubnika best live casino games
| #
Clubnika Casino is the place for true gambling enthusiasts, where everyone finds their spot.
Here, you’ll find the best games, profitable offers, and
unique opportunities to win. We guarantee the safety, fairness, and transparency of all gaming processes.
Why is Clubnika live dealer games your ideal choice?
By joining Clubnika Casino, you gain access to generous
bonuses, free spins, and regular promotions that
will significantly enhance your chances of success.
At Clubnika Casino, you can be confident that any questions will
be resolved promptly thanks to our round-the-clock support.
When is the best time to join Clubnika Casino? Don’t waste
any time, start right now and get bonuses that will speed up your
journey to victory. Here’s what awaits you at Clubnika Casino:
Generous bonuses for newcomers and regular free spins.
Tournaments with big prizes and incredible winning opportunities.
Game updates every month so you’ll never get bored.
Join Clubnika Casino and become part of the thrilling world of winnings!. https://clubnika-casinoeuphoria.website/
Rabyitabe
| #
darknet marketplace darkmarket link
source
| #
I am not sure where you are getting your info, but good topic.
I needs to spend some time learning more or understanding more.
Thanks for fantastic info I was looking for this information for my mission.
drenajnie raboti spb_eust
| #
смета на устройство дренажа https://drenazh-uchastka-krasnodar.ru/ .
drenajnie raboti spb_vbel
| #
дренаж под ключ в ленинградской области дренаж под ключ в ленинградской области .
WilliamIRrutty
| #
darknet drug market darknet market
Pingrutty
| #
dark market dark markets
DonDonfaita
| #
dark market url dark markets 2025
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
Ronaldbut
| #
МикоФарм Фунги
1win_diMr
| #
один вин http://www.1win6002.ru .
Starda Casino
| #
Welcome to Starda Casino – the best place for gambling entertainment! Here, you’ll find top-tier entertainment from renowned studios. https://starda-rush.top/ and get a chance to hit the jackpot!
What makes our casino special?
Popular games with high RTP.
Exclusive offers with free spins and cashback.
Instant transactions without hidden fees.
Modern design for a seamless experience.
Round-the-clock assistance answering within minutes.
Start playing now and experience the ultimate excitement!
Kxyufaita
| #
darknet markets darkmarket list
FNDaviditabe
| #
dark web market list darkmarket link
mostbet_qdOt
| #
мостюет mostbet6007.ru .
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
Monitoring transporta_iiOi
| #
установка мониторинга транспорта http://kontrol-avto.ru .
Donaldlaf
| #
dark markets 2025 dark market 2025
drenajnie raboti spb_wqst
| #
дренаж санкт петербург http://drenazh-uchastka-krasnodar.ru .
MarkNOlaf
| #
dark web market darknet drugs
Toliknaf
| #
darknet links darknet drug market
drenajnie raboti spb_bqel
| #
дренажные системы спб дренажные системы спб .
RobertDaw
| #
Non-Gamstop casino sites feel so much more relaxed—big thumbs up!
non.gamstop casinos
1win_hdot
| #
1wi. http://www.alfatraders.borda.ru/?1-0-0-00004932-000-0-0-1743258210 .
Rabyitabe
| #
bitcoin dark web dark web sites
WilliamIRrutty
| #
darkmarket link darknet markets onion address
Pingrutty
| #
darkmarket link onion dark website
CalowlaW
| #
На сайте https://boostclicks.ru/ вы сможете ознакомиться с рекомендациями, ценными и важными советами про API интеграцию, скрипты, а также любопытные сервисы из мира трафика. Для того чтобы вам было удобней ориентироваться, все материалы поделены на разделы, что позволит отыскать то, что действительно актуально и интересно в данный момент. Регулярно на портале появляется новый, интересный контент на данную тему. Имеется и список тех статей, которые пользуются особой популярностью. Все они созданы лучшими и высококлассными экспертами с огромным опытом.
1win_wlMr
| #
1win ru https://1win6002.ru/ .
Kxyufaita
| #
dark markets darknet drug market
1win_ioMl
| #
1 win.com 1win6049.ru .
mostbet_ypOt
| #
мостбет скачать на андроид https://mostbet6007.ru/ .
DonDonfaita
| #
dark market onion dark website
Ronaldbut
| #
МикоФарм Фунги
1win_dxOi
| #
1 вин. balashiha.myqip.ru/?1-12-0-00000437-000-0-0-1743258848 .
avtolombardkemerovosig
| #
автоломбард под птс в кемерово
https://es-africa.com/employer/newrba6811/
кредит под залог птс
Monitoring transporta_svOi
| #
купить gps глонасс мониторинг транспорта купить gps глонасс мониторинг транспорта .
MarkNOlaf
| #
darknet markets darkmarket 2025
Donaldlaf
| #
darknet site dark market link
WilliamIRrutty
| #
darkmarket url dark websites
Toliknaf
| #
dark market list dark web market links
RobertDaw
| #
Non-Gamstop casinos make gaming feel unrestricted—highly recommend!
non gamstop no deposit casino
Pingrutty
| #
dark web marketplaces darknet drug links
Kxyufaita
| #
darknet links dark markets 2025
aksjdf_lit
| #
Witamy w SlottyWay Kasyno – wiodącej, w pełni licencjonowanej platformie gier online, łączącej najnowocześniejsze rozwiązania technologiczne z doskonałą i przyjazną obsługą klienta. Nasza biblioteka gier jest niezwykle bogata i zróżnicowana – od klasycznych automatów z klimatem tradycyjnych kasyn, przez najnowsze sloty wideo, aż po nowoczesne gry stołowe na żywo z krupierami, które zapewnią Ci wrażenia rodem z prestiżowych salonów gier: Slottyway Vladimir dolboeb. Stawiamy na pełne bezpieczeństwo i transparentność. Nasze zaawansowane systemy ochrony, w połączeniu z szyfrowaniem SSL, gwarantują ochronę Twoich danych osobowych i finansowych. Współpracujemy wyłącznie z uznanymi dostawcami oprogramowania i stosujemy rygorystyczne standardy bezpieczeństwa.
Jamessok
| #
לאהוב. אתם תזיינו אותי? – כן, מותק, אנחנו הולכים לזיין אותך כמו שצריך. – אנחנו הולכים לקחת אותך בדרך הבוגרת. רק את צריכה בדירה לולדימיר בדירה דיסקרטית. מה דעתך? התחת היפה של אשתך! ולדימיר ברוסלן בתחינה, והוא לא איחרה לחכות. לאחר שראה את המבט הזה כהזמנה, read content
Rabyitabe
| #
dark market 2025 dark web market list
Monitoring transporta_szOi
| #
контроль транспорта цена контроль транспорта цена .
MarkNOlaf
| #
darknet drug links darknet marketplace
Ronaldbut
| #
https://mycofarm.ru/
DonDonfaita
| #
dark web market links darknet sites
WilliamIRrutty
| #
best darknet markets darknet markets
Donaldlaf
| #
darkmarket darknet markets onion
FNDaviditabe
| #
darknet markets links darknet marketplace
Pingrutty
| #
darknet drugs darknet markets 2025
RobertDaw
| #
Non Gamstop casino UK support is top-notch—really impressed!
non gamstop casinos uk 10 deposit
Toliknaf
| #
darkmarket url onion dark website
MegexBox
| #
На сайте https://stakecasino.tech изучите подробную, актуальную информацию, которая касается популярного онлайн-казино «Stake Casino». Это место для любителей азарта, ведь здесь есть еще и букмекерская контора. Каждому игроку предлагается огромное количество интересных возможностей для того, чтобы получить море приятных, положительных эмоций. А заодно вы сможете заработать много денег на все, что пожелаете. Вас порадует понятный, простой интерфейс, а также огромное количество бонусов, безупречная защита.
nevedCar
| #
Adguard-faq.com предоставляет информацию. Разъясним, что значит, подписка на Адгуард VPN и как управлять ею. Расскажем, где на AdGuard можно взять промокоды. Работа с Adguard имеет много положительных сторон. На сайте рассмотрим самые очевидные. Ознакомьтесь уже сейчас с ответами на часто задаваемые вопросы. https://adguard-faq.com – здесь расскажем, зачем нужны официальные сайты-зеркала продуктов AdGuard. Узнаете, как купить продукты экосистемы приватности Адгуард. Ищите отменный блокировщик рекламы? Adguard – отличный выбор для вас!
avtolombardmsksig
| #
автоломбард авто
https://analyticsjobs.in/jobs/companies/avtolombard-118791/
взять кредит под птс
MarkNOlaf
| #
darknet sites darknet markets onion address
Rabyitabe
| #
darkmarket link darknet websites
1win_uqMl
| #
1win официальный сайт 1win официальный сайт .
ZavamlReK
| #
Продать технику легко, обратитесть в скупку78. Компания https://spbskupka78.ru/ занимает лидирующие позиции по выкупу устройств и техники у населения. В данный момент компания ввела оценку через мессенджеры whats app и телеграмм. Наша компания добавила в прайс оценку таких устройства, как: айфоны от 7 до 16 модели, iPad от 2014 года до 2024 (процессор M4), макбуки от 2012 года до 2024 на процессоре М4, ноутбуки бизнес сегмента и игровые, аудиосистемы, микрофоны, ресиверы и усилители звука, телевизоры и мониторы.
Ronaldbut
| #
https://mycofarm.ru/
Jamessok
| #
הכנת דו ” ח? הבחור נופף בידו וצחק. – אתה מדבר על זה… לא, כמובן. כשעלה שני נשפים התמונות בעצמי, אז החלטתי לזרוק למקס עוד כמה את זה ככה, בלי להסיט את מבטו. בהתחלה הוא היה ביישן…. אבל אז העז…. תפס אותי בחלק האחורי של הראש ויירט את היוזמה. פטרובנה היא נערות ליווי ברחובות
Lazrbrd
| #
Где заказать диплом по нужной специальности?
Приобрести диплом ВУЗа по невысокой стоимости возможно, обращаясь к проверенной специализированной фирме.: prodiplome.com
WilliamIRrutty
| #
dark markets dark market
DonDonfaita
| #
darknet markets darknet markets onion
WilliamDix
| #
Мы изготавливаем дипломы любых профессий по доступным ценам. Дипломы производятся на настоящих бланках государственного образца Заказать диплом ВУЗа diplom-onlinex.com
Kxyufaita
| #
darknet market links dark websites
Pingrutty
| #
darknet markets onion address darknet drugs
1win_gtOi
| #
1win зайти https://www.balashiha.myqip.ru/?1-12-0-00000437-000-0-0-1743258848 .
Donaldlaf
| #
dark websites darknet market list
GohifeFer
| #
На сайте https://xn—-7sbecpcasfm0beeaecirc5b3a2g.kz/ получите расчет на то, во сколько вам обойдется корпоративное обучение. «CASPIAN TRAINING GROUP» более 20 лет оказывает помощь в том, чтобы вы прошли успешное обучение. Для того чтобы получить консультацию, коммерческое предложение, необходимо ввести в специальную форму свои личные данные, телефон, электронную почту. Компания оказывает такие услуги, как: бизнес-тренинги, обучение персонала языкам, бизнес-симуляции. В этой компании ни один десяток предприятий проходит обучение за год.
1win_blMl
| #
1win официальный сайт скачать http://1win6049.ru/ .
MarkNOlaf
| #
darkmarket link dark market url
RobertDaw
| #
Non Gamstop casinos no deposit offers keep me playing—awesome!
non gamstop casinos uk betz.casino
Toliknaf
| #
darknet drug store darknet drug store
Rabyitabe
| #
dark market onion tor drug market
WilliamIRrutty
| #
darkmarket 2025 darknet markets
Kxyufaita
| #
dark web market list dark markets 2025
FNDaviditabe
| #
darkmarket url onion dark website
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
Ronaldbut
| #
МикоФарм Фунги
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
Pingrutty
| #
onion dark website onion dark website
Josephkaw
| #
последние спортивные новости
DonDonfaita
| #
darknet markets links dark market 2025
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
avtolombardmsksig
| #
займ под залог автоломбард
https://cdltruckdrivingcareers.com/employer/ishayoga7336/
кредит под птс авто
MarkNOlaf
| #
darknet drugs darknet site
w69aias
| #
ติดตั้งแอป w69aias รับประสบการณ์เดิมพันที่เหนือกว่า พร้อมฟีเจอร์ใหม่ช่วยให้เล่นเกมได้ง่ายขึ้น
w69 slot asia login
| #
ดาวน์โหลดแอป w69 slot asia login
แล้วสนุกกับสล็อต แจ็คพอตแตกง่าย และอัตราจ่ายที่ดีที่สุด
Donaldlaf
| #
dark web link darknet markets links
เกมส์ สล็อต w69 slot
| #
ดาวน์โหลดแอป เกมส์ สล็อต w69 slot – https://rxq58.com ได้ง่ายๆ
เพียงไม่กี่ขั้นตอน พร้อมรับโบนัสต้อนรับ 100$ สนุกกับการเดิมพันที่ดีที่สุดบนมือถือ
Bevaxowreave
| #
На сайте https://smartflow.ru вы сможете выбрать качественную, функциональную сантехнику для обустройства ванной комнаты в доме либо квартире. Ассортимент «SMARTFLOW» включает в себя такие изделия, которые выполнены из высококачественных и современных материалов, безупречного стиля. Все унитазы, биде, модульные ванные идеально впишутся в концепцию. Каждая вещь идеально сочетает надежность, прочность, а также изысканность. Для того чтобы выбрать что-то определенное, изучите галерею, ознакомьтесь и с особой системой смыва «Торнадо».
สล็อต ออนไลน์ w69
| #
ติดตั้งแอป สล็อต ออนไลน์ w69 แล้วรับการแจ้งเตือนแมตช์สำคัญ โปรโมชั่นพิเศษ
และข่าวสารก่อนใคร
RobertDaw
| #
Non Gamstop UK casino has a great feel—big win!
non gamstop casinos uk no deposit bonus
w69ทางเข้าหลัก
| #
แอป w69ทางเข้าหลัก รองรับระบบฝาก-ถอนที่รวดเร็ว ให้คุณทำธุรกรรมได้ง่ายดายเพียงปลายนิ้ว
WilliamIRrutty
| #
dark web sites darknet markets 2025
Toliknaf
| #
tor drug market darknet markets
w69con
| #
w69con ปรับแต่งอินเทอร์เฟซแอปใหม่
ดีไซน์สวยงาม ค้นหาง่าย และใช้งานได้สะดวกกว่าเดิม
JerryDab
| #
In Azerbaijan, the 12-day holiday vacation has ended https://pin.it/5XGTX4YRH
aksjdf_lit
| #
Witamy w SlottyWay Kasyno – wiodącej, w pełni licencjonowanej platformie gier online, łączącej najnowocześniejsze rozwiązania technologiczne z doskonałą i przyjazną obsługą klienta. Nasza biblioteka gier jest niezwykle bogata i zróżnicowana – od klasycznych automatów z klimatem tradycyjnych kasyn, przez najnowsze sloty wideo, aż po nowoczesne gry stołowe na żywo z krupierami, które zapewnią Ci wrażenia rodem z prestiżowych salonów gier: Slottyway Casino. Stawiamy na pełne bezpieczeństwo i transparentność. Nasze zaawansowane systemy ochrony, w połączeniu z szyfrowaniem SSL, gwarantują ochronę Twoich danych osobowych i finansowych. Współpracujemy wyłącznie z uznanymi dostawcami oprogramowania i stosujemy rygorystyczne standardy bezpieczeństwa.
w69 slot daftar
| #
w69 slot daftar ปรับปรุงฟังก์ชันแอปใหม่ ใช้งานง่ายกว่าเดิม สนุกกับคาสิโนสดและเกมสล็อตได้ทุกที่ทุกเวลา
Kxyufaita
| #
darknet drug store darknet markets onion address
FNDaviditabe
| #
darkmarket link dark web market list
Dnrtweg
| #
Где приобрести диплом специалиста?
Мы предлагаем дипломы любой профессии по выгодным ценам. Мы можем предложить документы техникумов, расположенных в любом регионе России. Можно приобрести диплом от любого заведения, за любой год, в том числе документы старого образца. Документы печатаются на “правильной” бумаге самого высокого качества. Это позволяет делать настоящие дипломы, не отличимые от оригинала. Они будут заверены всеми требуемыми печатями и подписями. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Для нас важно, чтобы документы были доступными для большого количества наших граждан. diplom-profi.ru/kupit-diplom-v-samare-2-4
Diplomi_nzKt
| #
Купить диплом любого ВУЗа!
Мы готовы предложить документы институтов, которые находятся в любом регионе Российской Федерации.
diplomj-irkutsk.ru/kupit-attestat-za-9-klass-v-moskve-4
w69 app login
| #
ติดตั้งแอป w69 app login รับประสบการณ์เดิมพันที่เหนือกว่า พร้อมฟีเจอร์ใหม่ช่วยให้เล่นเกมได้ง่ายขึ้น
Josephkaw
| #
новости футбола сегодня
w69 login slot
| #
ติดตั้งแอป w69 login slot – https://zfqc3.com รับประสบการณ์เดิมพันที่เหนือกว่า พร้อมฟีเจอร์ใหม่ช่วยให้เล่นเกมได้ง่ายขึ้น
Sazrsrk
| #
Мы готовы предложить дипломы любой профессии по доступным ценам. Цена может зависеть от той или иной специальности, года выпуска и образовательного учреждения. Стараемся поддерживать для заказчиков адекватную политику цен. Важно, чтобы документы были доступны для большого количества наших граждан. купить диплом в тюмени
w69 mo
| #
w69 mo – https://kmib5.com ปรับปรุงฟังก์ชันแอปใหม่ ใช้งานง่ายกว่าเดิม สนุกกับคาสิโนสดและเกมสล็อตได้ทุกที่ทุกเวลา
Trefoks
| #
Купить документ института вы имеете возможность у нас в Москве. diplomc-v-ufe.ru/kupit-diplom-omsk
Rabyitabe
| #
dark web market darknet site
เว็บw69
| #
อัปเกรดแอป เว็บw69 วันนี้ เพื่อการเล่นเกมที่ราบรื่นกว่าเดิม พร้อมฟังก์ชันใหม่ที่ช่วยให้คุณสนุกยิ่งขึ้น
uşak taksi
| #
Your site is very nice, the articles are great, I wish you continued success, I love your site very much, I will visit it constantly
Pingrutty
| #
dark websites dark market onion
w69 top
| #
ติดตั้งแอป w69 top แล้วพบกับการแจ้งเตือนโปรโมชั่นและกิจกรรมใหม่ๆ ก่อนใคร
w69slotทางเข้า
| #
ดาวน์โหลดแอป w69slotทางเข้า ง่ายๆ เพียงไม่กี่คลิก สนุกกับการเดิมพันกีฬา
คาสิโน และสล็อตในที่เดียว
Ronaldbut
| #
МикоФарм Фунги
Jariorroa
| #
Где приобрести диплом по актуальной специальности?
Наши специалисты предлагают выгодно и быстро заказать диплом, который выполнен на оригинальном бланке и заверен печатями, водяными знаками, подписями официальных лиц. Диплом способен пройти лубую проверку, даже с использованием специальных приборов. Достигайте свои цели быстро с нашим сервисом.
Заказать диплом университета diplomt-v-chelyabinske.ru/kupit-diplom-v-bryanske-15/
Sazrzpi
| #
купить диплом политеха
w69 ถอนเงิน ไม่ได้
| #
สมัครสมาชิกผ่านแอป w69 ถอนเงิน ไม่ได้ รับโบนัสต้อนรับ 100$ และสิทธิพิเศษอีกมากมาย
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Партнерство с нашей компанией гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
С широким ассортиментом редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым текущие трудности превращаются в завтрашние возможности.
Наша продукция:
Мишень гафния
MarkNOlaf
| #
onion dark website darknet markets links
Jariorgtu
| #
Где приобрести диплом по нужной специальности?
Наша компания предлагает максимально быстро заказать диплом, который выполняется на оригинальном бланке и заверен мокрыми печатями, водяными знаками, подписями должностных лиц. Документ способен пройти любые проверки, даже с использованием профессиональных приборов. Решайте свои задачи быстро с нашим сервисом. Приобрести диплом любого ВУЗа! vseamoskva.flybb.ru/viewtopic.php?f=2&t=1120
DonDonfaita
| #
dark market onion dark market onion
EdwardBiorn
| #
Ткань “дышит”, не жарко!
женские костюмы томск
Mazrdnh
| #
Мы предлагаем дипломы психологов, юристов, экономистов и любых других профессий по выгодным ценам. Стараемся поддерживать для клиентов адекватную ценовую политику. Важно, чтобы документы были доступными для большого количества наших граждан.
Покупка документа, который подтверждает обучение в ВУЗе, – это выгодное решение. Купить диплом ВУЗа: diplomius-docs.com/kupit-diplom-texnikuma-ob-okonchanii-3/
Lazrbpv
| #
Приобрести диплом ВУЗа!
Мы можем предложить дипломы любой профессии по приятным тарифам— diplomgorkiy.com/kupit-diplom-o-srednem-meditsinskom-obrazovanii-bistro-2/
SamuelGet
| #
Postanowilem sprawdzic aseita – bylem zawiedziony natychmiast. Zasoby edukacyjne sa przestarzale, nauczyciele nawet nie reaguja na zgloszenia. Pieniadze zostaly pobrane od razu, a rzetelnej edukacji zupelnie nie uzyskalem. Obsluga klienta to prawdziwy dramat, czuje, ze tam siedza bezduszne systemy. Bezwzgledne oszustwo, odradzam absolutnie nikomu! Lepiej zainwestowac srodki na kurs wartosciowego, a nie na podobny smiec.
ekokixHon
| #
Chat-gptru – лаборатория умной нейросети. Загрузите фотографию – ИИ мгновенно определит, что на ней изображено, и предоставит пояснения либо рекомендации. GPT быстро подбирает необходимую информацию из актуальных источников. https://chat-gptru.com – тут с мнениями наших пользователей можете ознакомиться. Также здесь указаны цены. Для применения нейросети нужно в чат ввести ваш запрос и отправить нажать. Chat GPT генерирует тексты для любых целей, придерживается высоких стандартов безопасности и конфиденциальности.
WilliamIRrutty
| #
darkmarket list dark web sites
Jamietah
| #
Переделанные счетчики
FNDaviditabe
| #
dark market list darknet market lists
Kxyufaita
| #
bitcoin dark web darknet drug store
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог высококачественных изделий из ценных материалов. Не важно, какие объемы вам необходимы – от мелких партий до обширных поставок, мы обеспечиваем быстрое выполнение вашего заказа.
Каждая единица товара подтверждена соответствующими документами, подтверждающими их качество. Превосходное обслуживание – наш стандарт – мы на связи, чтобы улаживать ваши вопросы и предоставлять решения под особенности вашего бизнеса.
Доверьте вашу потребность в редких металлах профессионалам РедМетСплав и убедитесь в гибкости нашего предложения
поставляемая продукция:
Проволока висмутовая L54930 – UNS Проволока висмутовая L54930 – UNS является высококачественным материалом, используемым РІ различных промышленных Рё научных приложениях. Рзготавливается РёР· висмута, что гарантирует отличные антикоррозионные свойства Рё устойчивость Рє высоким температурам. Рта проволока идеальна для сварки, создания сплавов Рё использования РІ электротехнике. Благодаря СЃРІРѕРёРј уникальным характеристикам, РѕРЅР° находит применение РІ медицинских устройствах Рё электронике. Если РІС‹ хотите купить Проволока висмутовая L54930 – UNS, выбирайте надежных поставщиков, чтобы получить РїСЂРѕРґСѓРєС‚ наилучшего качества.
RobertDaw
| #
Found the best non Gamstop casino for blackjack—loving it so far!
non gamstop ukgc casino sites
WilliamDix
| #
Мы готовы предложить дипломы любых профессий по приятным тарифам. Дипломы производятся на подлинных бланках государственного образца Приобрести диплом ВУЗа diplomers.com
Diplomi_zoka
| #
купить диплом в тобольске diplomys-vsem.ru .
Gefevpek
| #
Visit https://bestcasinoideal.com/ and discover expert reviews, top strategies and essential tips. Explore online slots, table games, live dealer experiences. We have compiled a complete collection of the best online casinos and gambling sites for 2025, tested and rated by experts.
Toliknaf
| #
darkmarket 2025 dark web market links
Pingrutty
| #
darknet market list darkmarkets
avtolombardnovosibsig
| #
автоломбард птс
https://career.abuissa.com/employer/zaim-pod-zalog-pts-12174/
кредит залог птс наличные
zaimcaree
| #
Быстрые онлайн займы на карту, подробнее на официальном сайте на сайте
https://wiki.cosmaticdrift.net/view/Cs2case_16k
| #
Что такое “Кейсы КС” и “Кейсы КС ГО”?
“Кейсы КС” (или “Кейсы CS”) и “Кейсы КС ГО” (CS:GO) — это виртуальные контейнеры, которые используются в играх Counter-Strike (КС), включая Counter-Strike: Global Offensive (CS:GO) и его обновленную версию Counter-Strike 2 (CS2). Внутри этих кейсов находятся различные игровые предметы — скины для оружия, ножи, перчатки и другие косметические элементы, которые игроки могут получить и использовать в игре для украшения своего инвентаря.
Как это работает?
1. **Регистрация**: Вы заходите на сайт и регистрируетесь, обычно через Steam, чтобы связать свой игровой аккаунт.
2. **Пополнение баланса**: Нужно внести деньги на сайт (например, через карту, криптовалюту или электронные кошельки), чтобы купить кейсы или внутриигровую валюту.
3. **Выбор кейса**: На сайте представлены разные кейсы с указанием возможных наград (от дешевых скинов до редких ножей) и их стоимости.
4. **Открытие кейса**: Вы выбираете кейс, оплачиваете его и открываете. Система случайным образом определяет, какой предмет вы получите.
5. **Вывод выигрыша**: Если вам повезло, вы можете вывести скин в Steam и использовать его в игре.
Чем отличается “Кейсы КС” от “Кейсы КС ГО”?
– **Кейсы КС**: Общий термин, который может относиться к кейсам в любой версии Counter-Strike, включая CS2.
– **Кейсы КС ГО**: Конкретно кейсы из CS:GO, которые были популярны до выхода CS2. Теперь многие платформы, адаптируют их под CS2, но скины из CS:GO все еще совместимы с новой игрой.
Если ты хочешь попробовать, начни с малого — внеси небольшую сумму и протестируй, как все работает. Например, выбери дешевый кейс и посмотри, что выпадет. Это поможет понять механику без больших рисков.
https://ai-db.science/wiki/Cs2case_93F
Sazrbjf
| #
Мы можем предложить дипломы любой профессии по выгодным ценам.– kupit-diplom24.com/kupit-diplom-v-moskve-s-zaneseniem-v-reestr-kachestvenno/
Rabyitabe
| #
darkmarket list dark web market urls
StevenJutle
| #
http://www.odnopolchane.net/forum/group.php?do=discuss&groupid=79&discussionid=688&gmid=2963
Sazraov
| #
Приветствую!
Купить диплом ВУЗа по выгодной стоимости возможно, обратившись к надежной специализированной фирме. Заказать диплом о высшем образовании: kupitediplom0027.ru/kupit-diplom-yurista-8/
www w69
| #
วิธีติดตั้งแอป www w69
บนมือถือ ใช้เวลาเพียงไม่กี่นาที รองรับทุกระบบ ให้คุณเริ่มเดิมพันได้ทันที
Sazrmka
| #
Хочу поделиться своим опытом по заказу аттестата ПТУ. Думал, что это невозможно, и начал искать информацию в интернете по теме: диплом об окончании класса купить, купить диплом свидетельство, купить диплом ссср в ростове, купить диплом 2000, купить диплом в нурсултане. Постепенно углубляясь в тему, нашел отличный ресурс здесь: proffdiplomik.com/sochi
w69สล็อต
| #
อัปเกรดแอป w69สล็อต วันนี้ เพื่อการเล่นเกมที่ราบรื่นกว่าเดิม พร้อมฟังก์ชันใหม่ที่ช่วยให้คุณสนุกยิ่งขึ้น
w69เข้า
| #
ดาวน์โหลดแอป w69เข้า แล้วสนุกกับสล็อต แจ็คพอตแตกง่าย และอัตราจ่ายที่ดีที่สุด
Volodyanaf
| #
bitcoin dark web darknet market links
w69cy
| #
เล่นเกม w69cy – https://bavr3.com ผ่านแอป สนุกได้ทุกที่ พร้อมอัปเดตเกมใหม่ให้คุณได้สัมผัสก่อนใคร
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы обеспечить любой запрос с непревзойденным обслуживанием.
Наша команда поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – наилучшее решение для вашего бизнеса.
Наша продукция:
Кольцо РёР· драгоценных металлов платиновое 9С…3С…4.5 РјРј ПлПд95-5 РўРЈ РЁРёСЂРѕРєРёР№ выбор высококачественных кольц РёР· драгоценных металлов. Рлегантные украшения, созданные талантливыми мастерами. Уникальность Рё изысканность РІ каждой детали. Возможность заказа персонального кольца РїРѕ вашим пожеланиям. Подчеркните СЃРІРѕСЋ индивидуальность СЃ нашими кольцами.
w69 ทางเข้าเล่น
| #
โหลดแอป w69 ทางเข้าเล่น รับประสบการณ์การเล่นเกมที่สมจริงกว่าเดิม ด้วยระบบถ่ายทอดสดแบบ HD
w69 online casino
| #
อินเทอร์เฟซใหม่ของแอป w69 online casino
ช่วยให้การนำทางลื่นไหลขึ้น ไม่ต้องเสียเวลาค้นหาเกมโปรดของคุณ
rli59.com
| #
ติดตั้งแอป w69 – rli59.com – แล้วพบกับการแจ้งเตือนโปรโมชั่นและกิจกรรมใหม่ๆ ก่อนใคร
Mazrdsx
| #
Где приобрести диплом специалиста?
Готовый диплом с нужными печатями и подписями полностью отвечает запросам и стандартам Министерства образования и науки РФ, неотличим от оригинала. Не следует откладывать свои мечты и цели на несколько лет, реализуйте их с нашей помощью – отправьте простую заявку на изготовление диплома уже сегодня! Получить диплом о высшем образовании – быстро и легко! freediplom.com/kupit-diplom-11-klassa-bistro-i-nadezhno-2/
DonDonfaita
| #
darknet markets url dark web link
Mazrbkz
| #
Где приобрести диплом специалиста?
Мы предлагаем документы об окончании любых университетов РФ. Документы производят на подлинных бланках государственного образца. pashasevkav.getbb.ru/posting.php?mode=post&f=31&sid=af6e328650cc9cccafb5e3f34a0a6528
Uazrhss
| #
Добрый день!
Для определенных людей, купить диплом университета – это необходимость, возможность получить достойную работу. Но для кого-то – это желание не терять огромное количество времени на учебу в институте. Что бы ни толкнуло вас на такой шаг, наша компания готова помочь. Быстро, качественно и по разумной стоимости сделаем диплом нового или старого образца на государственных бланках со всеми печатями.
Основная причина, почему многие прибегают к покупке документов, – желание занять определенную работу. Например, способности и опыт позволяют кандидату устроиться на привлекательную работу, а документального подтверждения квалификации не имеется. При условии, что работодателю важно присутствие “корочек”, риск потерять хорошее место довольно высокий.
Приобрести документ института можно у нас. Мы предлагаем документы об окончании любых ВУЗов России. Вы получите диплом по любой специальности, включая документы СССР. Даем 100% гарантию, что в случае проверки документов работодателем, подозрений не появится.
Ситуаций, которые вынуждают приобрести диплом ВУЗа достаточно. Кому-то срочно требуется работа, в результате необходимо произвести впечатление на руководителя при собеседовании. Некоторые хотят попасть в престижную компанию, чтобы повысить свой статус в обществе и в последующем начать свой бизнес. Чтобы не терять попусту годы жизни, а сразу начинать успешную карьеру, применяя имеющиеся навыки, можно купить диплом прямо в интернете. Вы сможете быть полезным для социума, обретете финансовую стабильность в максимально короткие сроки- купить диплом техникума
เว็บสล็อตw69
| #
เว็บสล็อตw69 อัปเดตแอปใหม่ให้รองรับการเดิมพันแบบเรียลไทม์ เชียร์ทีมโปรดของคุณได้แบบไม่มีสะดุด
Sazrqys
| #
Заказ документа о высшем образовании через надежную компанию дарит ряд преимуществ. Это решение дает возможность сэкономить как дорогое время, так и серьезные финансовые средства. Тем не менее, на этом выгоды не ограничиваются, преимуществ гораздо больше.Мы изготавливаем дипломы любых профессий. Дипломы изготавливаются на подлинных бланках государственного образца. Доступная цена по сравнению с серьезными тратами на обучение и проживание в чужом городе. Заказ диплома института станет выгодным шагом.
Заказать диплом: diplom-club.com/kupite-meditsinskij-diplom-nadezhno-i-bistro/
Sazrpjc
| #
Мы предлагаем дипломы любых профессий по выгодным ценам. О преимуществах покупки документов в нашем сервисе
Вы заказываете диплом через надежную и проверенную фирму. Такое решение позволит вам сэкономить не только много денежных средств, но и ваше драгоценное время.
Плюсов гораздо больше:
• Дипломы печатаются на настоящих бланках со всеми печатями;
• Предлагаем дипломы любого ВУЗа РФ;
• Цена в разы меньше нежели довелось бы заплатить на очном и заочном обучении в университете;
• Доставка как по столице, так и в любые другие регионы России.
Заказать диплом о высшем образовании– http://babygirls001.copiny.com/question/details/id/1061062/ – babygirls001.copiny.com/question/details/id/1061062
w69thai
| #
ติดตั้งแอป w69thai แล้วรับการแจ้งเตือนแมตช์สำคัญ
โปรโมชั่นพิเศษ และข่าวสารก่อนใคร
Ronaldbut
| #
https://mycofarm.ru/
vidnovskoe
| #
Кладбище в Видном vidnovskoe актуальные данные о захоронениях, помощь в организации похорон, услуги по благоустройству могил. Схема проезда, часы работы и контактная информация.
Cazrhny
| #
Приветствую!
Мы изготавливаем дипломы любой профессии по разумным тарифам. Цена зависит от выбранной специальности, года получения и образовательного учреждения: п»їtutdiploms.com/
http://classicalmusicmp3freedownload.com/ja/index.php?title=:MilagroD00
| #
Что такое “Кейсы КС” и “Кейсы КС ГО”?
“Кейсы КС” (или “Кейсы CS”) и “Кейсы КС ГО” (CS:GO) — это виртуальные контейнеры, которые используются в играх Counter-Strike (КС), включая Counter-Strike: Global Offensive (CS:GO) и его обновленную версию Counter-Strike 2 (CS2). Внутри этих кейсов находятся различные игровые предметы — скины для оружия, ножи, перчатки и другие косметические элементы, которые игроки могут получить и использовать в игре для украшения своего инвентаря.
Как это работает?
1. **Регистрация**: Вы заходите на сайт и регистрируетесь, обычно через Steam, чтобы связать свой игровой аккаунт.
2. **Пополнение баланса**: Нужно внести деньги на сайт (например, через карту, криптовалюту или электронные кошельки), чтобы купить кейсы или внутриигровую валюту.
3. **Выбор кейса**: На сайте представлены разные кейсы с указанием возможных наград (от дешевых скинов до редких ножей) и их стоимости.
4. **Открытие кейса**: Вы выбираете кейс, оплачиваете его и открываете. Система случайным образом определяет, какой предмет вы получите.
5. **Вывод выигрыша**: Если вам повезло, вы можете вывести скин в Steam и использовать его в игре.
Чем отличается “Кейсы КС” от “Кейсы КС ГО”?
– **Кейсы КС**: Общий термин, который может относиться к кейсам в любой версии Counter-Strike, включая CS2.
– **Кейсы КС ГО**: Конкретно кейсы из CS:GO, которые были популярны до выхода CS2. Теперь многие платформы, адаптируют их под CS2, но скины из CS:GO все еще совместимы с новой игрой.
Если ты хочешь попробовать, начни с малого — внеси небольшую сумму и протестируй, как все работает. Например, выбери дешевый кейс и посмотри, что выпадет. Это поможет понять механику без больших рисков.
https://opensourcebridge.science/wiki/Cs2case_3C
Eanrvof
| #
Для быстрого продвижения по карьерной лестнице понадобится наличие диплома ВУЗа. Приобрести диплом о высшем образовании у проверенной организации: diplomp-irkutsk.ru/kupit-diplom-o-polnom-srednem-obrazovanii-bistro-i-nadezhno/
Donaldlaf
| #
darknet drug market darkmarket link
Diplomi_raKt
| #
Купить диплом университета!
Мы предлагаем документы институтов, расположенных в любом регионе Российской Федерации.
diplomidlarf.ru/diplom-o-sredne-texnicheskom-obrazovanii-kupit-4
Pingrutty
| #
dark market url dark markets 2025
Lazranm
| #
Диплом любого ВУЗа России!
Без присутствия диплома очень нелегко было продвигаться вверх по карьерной лестнице. Именно из-за этого решение о заказе диплома стоит считать выгодным и целесообразным. Заказать диплом любого института uniqplacements.com/employer/gosznac-diplom-24
Dnrtmam
| #
Где приобрести диплом по актуальной специальности?
Мы предлагаем дипломы любых профессий по доступным ценам. Мы готовы предложить документы техникумов, расположенных на территории всей Российской Федерации. Можно купить диплом за любой год, указав подходящую специальность и хорошие оценки за все дисциплины. Документы выпускаются на “правильной” бумаге высшего качества. Это дает возможности делать государственные дипломы, которые невозможно отличить от оригинала. Они заверяются необходимыми печатями и штампами. Всегда стараемся поддерживать для заказчиков адекватную ценовую политику. Важно, чтобы документы были доступными для большого количества наших граждан. kupitediplom0029.ru/kupit-diplom-medbrata-2-6
Sazriyr
| #
Мы готовы предложить дипломы любых профессий по доступным тарифам. Стоимость может зависеть от конкретной специальности, года выпуска и образовательного учреждения. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Для нас важно, чтобы дипломы были доступны для подавляющей массы наших граждан. купить диплом училища во владивостоке
https://utahsyardsale.com/author/dantefloren/
| #
Что такое “Кейсы КС” и “Кейсы КС ГО”?
“Кейсы КС” (или “Кейсы CS”) и “Кейсы КС ГО” (CS:GO) — это виртуальные контейнеры, которые используются в играх Counter-Strike (КС), включая Counter-Strike: Global Offensive (CS:GO) и его обновленную версию Counter-Strike 2 (CS2). Внутри этих кейсов находятся различные игровые предметы — скины для оружия, ножи, перчатки и другие косметические элементы, которые игроки могут получить и использовать в игре для украшения своего инвентаря.
Как это работает?
1. **Регистрация**: Вы заходите на сайт и регистрируетесь, обычно через Steam, чтобы связать свой игровой аккаунт.
2. **Пополнение баланса**: Нужно внести деньги на сайт (например, через карту, криптовалюту или электронные кошельки), чтобы купить кейсы или внутриигровую валюту.
3. **Выбор кейса**: На сайте представлены разные кейсы с указанием возможных наград (от дешевых скинов до редких ножей) и их стоимости.
4. **Открытие кейса**: Вы выбираете кейс, оплачиваете его и открываете. Система случайным образом определяет, какой предмет вы получите.
5. **Вывод выигрыша**: Если вам повезло, вы можете вывести скин в Steam и использовать его в игре.
Чем отличается “Кейсы КС” от “Кейсы КС ГО”?
– **Кейсы КС**: Общий термин, который может относиться к кейсам в любой версии Counter-Strike, включая CS2.
– **Кейсы КС ГО**: Конкретно кейсы из CS:GO, которые были популярны до выхода CS2. Теперь многие платформы, адаптируют их под CS2, но скины из CS:GO все еще совместимы с новой игрой.
Если ты хочешь попробовать, начни с малого — внеси небольшую сумму и протестируй, как все работает. Например, выбери дешевый кейс и посмотри, что выпадет. Это поможет понять механику без больших рисков.
https://aexcom.org.pe/index.php/Cs2case_46S
Toliknaf
| #
darknet markets darknet market links
Kxyufaita
| #
darknet drugs darknet market list
zaimcaree
| #
Быстрые онлайн займы на карту, подробнее на официальном сайте ссылка
Sazrsqn
| #
Заказать диплом ВУЗа по выгодной стоимости можно, обращаясь к надежной специализированной фирме. Мы предлагаем документы об окончании любых ВУЗов России. Заказать диплом ВУЗа– diploma-groups24.ru/diplomy-po-specialnosti/diplom-farmacevta.html
Sazrgck
| #
купить аттестат об окончании 11 классов в калининграде
StevenJutle
| #
http://forum.ptia-avto.ru/viewtopic.php?f=16&t=1137
Volodyanaf
| #
bitcoin dark web dark web sites
Rabyitabe
| #
darkmarket url darknet markets onion address
drenajnie raboti spb_wxel
| #
дренаж участка под ключ цена дренаж участка под ключ цена .
drenajnie raboti spb_wrst
| #
дренажно распределительная система дренажно распределительная система .
https://indigenouspedia.com/index.php?title=User:Oliva1833648762
| #
Что такое “Кейсы КС” и “Кейсы КС ГО”?
“Кейсы КС” (или “Кейсы CS”) и “Кейсы КС ГО” (CS:GO) — это виртуальные контейнеры, которые используются в играх Counter-Strike (КС), включая Counter-Strike: Global Offensive (CS:GO) и его обновленную версию Counter-Strike 2 (CS2). Внутри этих кейсов находятся различные игровые предметы — скины для оружия, ножи, перчатки и другие косметические элементы, которые игроки могут получить и использовать в игре для украшения своего инвентаря.
Как это работает?
1. **Регистрация**: Вы заходите на сайт и регистрируетесь, обычно через Steam, чтобы связать свой игровой аккаунт.
2. **Пополнение баланса**: Нужно внести деньги на сайт (например, через карту, криптовалюту или электронные кошельки), чтобы купить кейсы или внутриигровую валюту.
3. **Выбор кейса**: На сайте представлены разные кейсы с указанием возможных наград (от дешевых скинов до редких ножей) и их стоимости.
4. **Открытие кейса**: Вы выбираете кейс, оплачиваете его и открываете. Система случайным образом определяет, какой предмет вы получите.
5. **Вывод выигрыша**: Если вам повезло, вы можете вывести скин в Steam и использовать его в игре.
Чем отличается “Кейсы КС” от “Кейсы КС ГО”?
– **Кейсы КС**: Общий термин, который может относиться к кейсам в любой версии Counter-Strike, включая CS2.
– **Кейсы КС ГО**: Конкретно кейсы из CS:GO, которые были популярны до выхода CS2. Теперь многие платформы, адаптируют их под CS2, но скины из CS:GO все еще совместимы с новой игрой.
Если ты хочешь попробовать, начни с малого — внеси небольшую сумму и протестируй, как все работает. Например, выбери дешевый кейс и посмотри, что выпадет. Это поможет понять механику без больших рисков.
https://idpedia.wiki/index.php/User:JessNeedham0
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Партнерство с нашей компанией гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа требуемого количества.
С широким ассортиментом редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Приступая к взаимодействию с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым сегодняшние вызовы превращаются в завтрашние возможности.
Наша продукция:
“ерамическая мишень CeO2, плоская форма
DonDonfaita
| #
darknet markets links dark web market urls
เวปw69
| #
อัปเดตแอป เวปw69 วันนี้เพื่อสัมผัสประสบการณ์ใหม่ ลื่นไหลกว่าเดิม ไม่มีสะดุด
Iariorgbl
| #
Приобрести документ института вы сможете в нашем сервисе. http://www.mamanija.lt/klausimai/18759/koks-mamu-forumas-jums-mielesnis
Mazrvbw
| #
Мы изготавливаем дипломы любой профессии по приятным тарифам. Стараемся поддерживать для клиентов адекватную ценовую политику. Для нас важно, чтобы дипломы были доступны для подавляющей массы граждан.
Покупка диплома, подтверждающего окончание института, – это выгодное решение. Приобрести диплом любого ВУЗа: diplommy.ru/diplom-srednee-spetsialnoe-obrazovanie-kupit-4/
slot w69
| #
เล่นบาคาร่า รูเล็ต และเกมไพ่อื่นๆ ผ่านแอป slot w69 ด้วยระบบที่ลื่นไหลและภาพคมชัดระดับ
HD
Kxyufaita
| #
dark markets 2025 dark web market list
Pingrutty
| #
darknet markets dark web sites
avtolombardnovosibsig
| #
выгодный кредит под залог авто
https://www.philthejob.nl/employer/retailjobacademy2814/
деньги под залог кредитного авто
w69ลิ้ง
| #
อัปเกรดแอป w69ลิ้ง วันนี้ เพื่อการเล่นเกมที่ราบรื่นกว่าเดิม พร้อมฟังก์ชันใหม่ที่ช่วยให้คุณสนุกยิ่งขึ้น
dshb7.com
| #
dshb7.com – https://dshb7.com ปรับปรุงอินเทอร์เฟซแอปให้ใช้งานง่ายขึ้น ดีไซน์สวยงาม ค้นหาเกมได้รวดเร็วและสะดวกสบาย
w69io
| #
w69io – https://qah64.com ปรับปรุงฟังก์ชันแอปใหม่ ใช้งานง่ายกว่าเดิม สนุกกับคาสิโนสดและเกมสล็อตได้ทุกที่ทุกเวลา
Donaldlaf
| #
onion dark website darkmarket list
Diplomi_lqKt
| #
Купить диплом о высшем образовании!
Мы предлагаем документы университетов, расположенных в любом регионе Российской Федерации.
poluchidiplom.com/mozhno-li-kupit-attestat-za-9-klass-4
w69คาสิโนออนไลน์
| #
ดาวน์โหลดแอป w69คาสิโนออนไลน์ ง่ายๆ เพียงไม่กี่คลิก สนุกกับการเดิมพันกีฬา คาสิโน และสล็อตในที่เดียว
w69สล็อต เครดิตฟรี
| #
ติดตั้งแอป w69สล็อต เครดิตฟรี – https://apmb1.com อย่างรวดเร็ว รองรับทั้ง iOS
และ Android สัมผัสประสบการณ์เดิมพันที่ลื่นไหลและสะดวกสบายกว่าเดิม
w69 bet เข้าสู่ระบบ
| #
อินเทอร์เฟซใหม่ของแอป w69 bet เข้าสู่ระบบ ช่วยให้การนำทางลื่นไหลขึ้น
ไม่ต้องเสียเวลาค้นหาเกมโปรดของคุณ
1win_ysMr
| #
1win зайти 1win6002.ru .
w69mobil
| #
w69mobil ปรับแต่งอินเทอร์เฟซแอปใหม่ ดีไซน์สวยงาม
ค้นหาง่าย และใช้งานได้สะดวกกว่าเดิม
Volodyanaf
| #
darknet market lists darknet markets 2025
StevenJutle
| #
https://wowlol.ru/forum/showthread.php?p=77714
Sheilaadurl
| #
РедМетСплав предлагает широкий ассортимент отборных изделий из редких материалов. Не важно, какие объемы вам необходимы – от небольших закупок до обширных поставок, мы гарантируем оперативное исполнение вашего заказа.
Каждая единица продукции подтверждена требуемыми документами, подтверждающими их качество. Опытная поддержка – наша визитная карточка – мы на связи, чтобы улаживать ваши вопросы и находить ответы под особенности вашего бизнеса.
Доверьте вашу потребность в редких металлах специалистам РедМетСплав и убедитесь в множестве наших преимуществ
Наша продукция:
РљСЂСѓРі магниевый РњРђ8 Фольга магниевая РњРђ8 – это идеальный выбор для ваших технических задач. РћРЅР° отличается высокой прочностью Рё отличной РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ стойкостью. Рспользуйте фольгу РІ производстве, РіРґРµ требуется высокая проводимость Рё теплоотведение. РЎ ее помощью можно добиться качественного соединения РІ электротехнике Рё РґСЂСѓРіРёС… отраслях. Преимущества: легкость, устойчивость Рє воздействию влаги, возможность использования РІ различных климатических условиях. Если РІС‹ ищете надежный Рё долговечный материал, вам стоит купить Фольга магниевая РњРђ8. Убедитесь РІ ее качествах уже сегодня!
Toliknaf
| #
darkmarket url darkmarket 2025
w69 เข้า สู่ ระบบ
| #
อัปเกรดแอป w69 เข้า สู่ ระบบ วันนี้ เพื่อการเล่นเกมที่ราบรื่นกว่าเดิม
พร้อมฟังก์ชันใหม่ที่ช่วยให้คุณสนุกยิ่งขึ้น
Kennethfem
| #
В нашем центре доступен ремонт разного уровня сложности аудиосистем Philips включая профилактику.
Преимущества нашего сервиса:
Профессиональная оценка проблемы
Оперативное устранение неисправностей
Доступные цены
Выезд мастера на дом по Москве и области
Получить подробную информацию или заказать консультацию вы можете на нашем сайте ремонт кофемашины philips saeco.
Доверьте свою технику профессионалам! Свяжитесь с нами по телефону – гарантируем результат!
mostbet_fmOt
| #
mostbet.kg http://www.mostbet6007.ru .
1win_qlot
| #
1win скачать kg http://alfatraders.borda.ru/?1-0-0-00004932-000-0-0-1743258210/ .
Cazruxe
| #
Получить диплом любого ВУЗа поможем. Купить аттестат в Тольятти – diplomybox.com/kupit-attestat-v-tolyatti
Dnrttaw
| #
Где купить диплом специалиста?
Мы изготавливаем дипломы любых профессий по приятным тарифам. Мы можем предложить документы техникумов, расположенных в любом регионе Российской Федерации. Можно заказать диплом за любой год, указав актуальную специальность и оценки за все дисциплины. Документы делаются на “правильной” бумаге самого высокого качества. Это позволяет делать настоящие дипломы, не отличимые от оригинала. Документы будут заверены необходимыми печатями и штампами. Всегда стараемся поддерживать для покупателей адекватную политику цен. Для нас очень важно, чтобы документы были доступны для большого количества граждан. okdiplom.ru/kupit-diplom-s-vneseniem-v-reestr-6
Sazrxdb
| #
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по доступным ценам. Цена может зависеть от выбранной специальности, года получения и ВУЗа. Стараемся поддерживать для заказчиков адекватную ценовую политику. Для нас очень важно, чтобы дипломы были доступны для подавляющей массы наших граждан. купить диплом в тольятти
zaimcaree
| #
Быстрые онлайн займы на карту, подробнее на официальном сайте ссылка
w69th
| #
ดาวน์โหลดแอป w69th – https://szc74.com ได้ง่ายๆ เพียงไม่กี่ขั้นตอน พร้อมรับโบนัสต้อนรับ 100$ สนุกกับการเดิมพันที่ดีที่สุดบนมือถือ
w69 คาสิโนออนไลน์
| #
w69 คาสิโนออนไลน์ พัฒนาแอปให้รองรับเกมมากขึ้น มีตัวเลือกหลากหลายให้คุณเดิมพันได้อย่างอิสระ
Rabyitabe
| #
darknet market lists darknet links
Ronaldbut
| #
https://mycofarm.ru/
Geraldnof
| #
https://plomba77.ru/plombi-dlja-plombiratora/plombi-trubchatie-aluminievye В мире защиты и контроля доступа, одноразовые пластиковые пломбы роторного типа занимают особое место. Их простота использования, надежность и визуальная индикация несанкционированного доступа делают их незаменимым инструментом для обеспечения безопасности различных объектов. Эти пломбы, изготовленные из высококачественного пластика, отличаются прочностью и устойчивостью к внешним воздействиям. Роторный механизм обеспечивает надежную фиксацию, а уникальный номер каждой пломбы позволяет вести учет и контроль.
Kxyufaita
| #
darknet links darknet marketplace
Sazrwsh
| #
купить официальный диплом о среднем образовании
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Закупка у нас гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Имея широкий спектр продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Приступая к взаимодействию с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым сегодняшние вызовы превращаются в завтрашние достижения.
Наши товары:
Гранулы высокочистого ванадия
Pingrutty
| #
dark market 2025 darknet site
betshah
| #
New Users, Register at betshah and Get a $100 Welcome Bonus
DonDonfaita
| #
darknet market list dark websites
https://droids-hack.ru/
| #
https://droids-hack.ru/ — это отличный способ
получить новые возможности.
Особенно если вы играете на мобильном устройстве с Android,
модификации открывают перед вами новые возможности.
Я нравится использовать взломанные игры, чтобы получать неограниченные ресурсы.
Моды для игр дают невероятную свободу выбора, что погружение
в игру гораздо красочнее. Играя с
плагинами, я могу добавить дополнительные функции, что добавляет
виртуальные путешествия и делает игру более захватывающей.
Это действительно невероятно,
как такие модификации могут улучшить переживания от игры, а при этом с максимальной безопасностью использовать такие модифицированные приложения можно без особых опасностей, если быть внимательным
и следить за обновлениями.
Это делает каждый игровой процесс лучше контролируемым,
а возможности практически выше всяких похвал.
Советую попробовать такие модифицированные версии для Android — это может добавить веселья в геймплей
drenajnie raboti spb_omel
| #
дренажные системы компании http://www.drenazh-uchastka-rostov.ru .
drenajnie raboti spb_zwst
| #
дренаж участка спб дренаж участка спб .
Volodyanaf
| #
darknet market links dark web market
Monitoring transporta_vlOi
| #
установка мониторинга автомобилей https://kontrol-avto.ru .
Donaldlaf
| #
dark market link darknet drug market
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог отборных изделий из ценных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до обширных поставок, мы обеспечиваем быстрое выполнение вашего заказа.
Каждая единица изделия подтверждена всеми необходимыми документами, подтверждающими их качество. Опытная поддержка – наша визитная карточка – мы на связи, чтобы разрешать ваши вопросы а также адаптировать решения под особенности вашего бизнеса.
Доверьте ваш запрос специалистам РедМетСплав и убедитесь в гибкости нашего предложения
Наши товары:
Кобальт Stellite 4LC Кобальт Stellite 4LC – это высококачественный сплав, разработанный для обеспечения прочности Рё сопротивления РёР·РЅРѕСЃСѓ. Рспользуется РІ различных отраслях, включая авиастроение Рё машиностроение. Благодаря своей высокой РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ стойкости Рё жаропрочности, этот материал прекрасно РїРѕРґС…РѕРґРёС‚ для работы РІ экстремальных условиях. Если вам нужен надежный материал для обработки Рё защиты деталей, РїРѕРєСѓРїРєР° Кобальт Stellite 4LC станет удачным решением. Рнвестируйте РІ качество Рё долговечность ваших изделий, выбрав Кобальт Stellite 4LC.
Kxyufaita
| #
darknet markets onion darknet websites
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Закупка у нас гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
Имея широкий спектр редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие всем нормам.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым сегодняшние вызовы превращаются в завтрашние возможности.
Наша продукция:
Высокочистые гранулы тантала
Toliknaf
| #
darknet markets links dark market link
Lazrulo
| #
Заказать диплом об образовании!
Мы предлагаем дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам— diplomg-cheboksary.ru/kupit-attestat-za-11-klass-v-moskve-bistro-i-udobno-8/
Jariorczp
| #
Где заказать диплом по нужной специальности?
Мы предлагаем выгодно и быстро приобрести диплом, который выполняется на оригинальной бумаге и заверен мокрыми печатями, штампами, подписями. Данный диплом способен пройти лубую проверку, даже с применением профессионального оборудования. Достигайте своих целей максимально быстро с нашим сервисом. Купить диплом о высшем образовании! publikacii.listbb.ru/viewtopic.php?f=3&t=2145
Zooma jackpot slots
| #
Welcome to Zooma Casino – a modern gaming platform with unique opportunities! Here, you’ll find exciting slot machines from world-class game providers. https://zooma-play.top/ to test your luck in top-rated games!
Why do thousands of players choose us?
A vast selection of games from industry leaders.
Loyalty programs on deposits and bets.
Instant withdrawals to convenient payment systems.
User-friendly design on any device.
24/7 support quickly resolving any issues.
Feel the thrill of gambling and take advantage of all benefits!
Mazrsov
| #
Мы изготавливаем дипломы любой профессии по приятным ценам. Всегда стараемся поддерживать для заказчиков адекватную политику цен. Для нас очень важно, чтобы дипломы были доступны для большого количества наших граждан.
Покупка диплома, подтверждающего обучение в университете, – это рациональное решение. Заказать диплом университета: diplom-bez-problem.com/kupit-diplom-vuza-nedorogo/
Iariorzen
| #
Купить документ о получении высшего образования вы сможете в нашем сервисе. sarasusa.dreamwidth.org/23915.html?mode=reply
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Работа с нами гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа требуемого количества.
Обладая обширной линейкой редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым текущие трудности превращаются в завтрашние достижения.
Наши товары:
Высокочистые плитки алюминия
Rabyitabe
| #
dark market link tor drug market
Pingrutty
| #
bitcoin dark web dark websites
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем внушительный выбор продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с непревзойденным обслуживанием.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете достоверность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – наилучшее решение для вашего бизнеса.
поставляемая продукция:
Лента бронзовая 0,12х190 мм БрОФ ГОСТ 1761-92 Покупайте качественные и универсальные ленты бронзовые от Редметсплав. Широкий выбор размеров и высокое качество материала. Надежное и стильное решение для ваших проектов.
DavidUrire
| #
Счетчики газа с пленкой Приборы учета с дистанционным управлением, электросчетчики, оснащенные пультом, устройства для измерения потребления электроэнергии с возможностью удаленного контроля, экономичные модели счетчиков, модифицированные приборы учета, разнообразные счетчики, газовые счетчики, подвергшиеся воздействию магнитом или пленкой, способы остановки работы счетчика, приобретение счетчика с пультом дистанционного управления, покупка электросчетчика с пультом.
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог отборных изделий из редких материалов. Не важно, какие объемы вам необходимы – от небольших закупок до крупных поставок, мы гарантируем оперативное исполнение вашего заказа.
Каждая единица товара подтверждена всеми необходимыми документами, подтверждающими их соответствие стандартам. Превосходное обслуживание – наш стандарт – мы на связи, чтобы разрешать ваши вопросы и предоставлять решения под требования вашего бизнеса.
Доверьте потребности вашего бизнеса специалистам РедМетСплав и убедитесь в широком спектре предлагаемых возможностей
Наши товары:
РџРѕРєРѕРІРєР° магниевая ISO-MgAl4Si – ISO 16220 Лист магниевый ISO-MgAl4Si – ISO 16220 – это высококачественный материал, выполненный РёР· магниевого сплава, который отличается отличными механическими свойствами Рё легкостью. Ртот лист идеально РїРѕРґС…РѕРґРёС‚ для различных промышленных применений, включая авиацию Рё автомобильное производство. Купить Лист магниевый ISO-MgAl4Si – ISO 16220 – значит обеспечить себя надежным Рё долговечным материалом, который прекрасно справляется СЃ нагрузками. РќРµ упустите возможность приобрести этот товар, оптимизировав СЃРІРѕРё бизнес-процессы СЃ помощью высокотехнологичных решений!
Volodyanaf
| #
dark market onion darknet market
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа любого необходимого объема.
С широким ассортиментом продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается обязательными документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым сегодняшние вызовы превращаются в завтрашние достижения.
Наши товары:
“ерамическая мишень CeO2, плоская форма
DonDonfaita
| #
darkmarket list bitcoin dark web
MichaelScOfs
| #
аренда квартир в ташкенте Ташкент, современная столица Узбекистана, привлекает своим сочетанием истории и динамичного развития. Для тех, кто планирует остаться здесь на длительный срок, вопрос аренды жилья становится первостепенным. Снять квартиру в Ташкенте – задача, требующая внимательного подхода и понимания местного рынка.
https://surgiteams.com/index.php/Cs2case_82H
| #
Что такое “Кейсы КС” и “Кейсы КС ГО”?
“Кейсы КС” (или “Кейсы CS”) и “Кейсы КС ГО” (CS:GO) — это виртуальные контейнеры, которые используются в играх Counter-Strike (КС), включая Counter-Strike: Global Offensive (CS:GO) и его обновленную версию Counter-Strike 2 (CS2). Внутри этих кейсов находятся различные игровые предметы — скины для оружия, ножи, перчатки и другие косметические элементы, которые игроки могут получить и использовать в игре для украшения своего инвентаря.
Как это работает?
1. **Регистрация**: Вы заходите на сайт и регистрируетесь, обычно через Steam, чтобы связать свой игровой аккаунт.
2. **Пополнение баланса**: Нужно внести деньги на сайт (например, через карту, криптовалюту или электронные кошельки), чтобы купить кейсы или внутриигровую валюту.
3. **Выбор кейса**: На сайте представлены разные кейсы с указанием возможных наград (от дешевых скинов до редких ножей) и их стоимости.
4. **Открытие кейса**: Вы выбираете кейс, оплачиваете его и открываете. Система случайным образом определяет, какой предмет вы получите.
5. **Вывод выигрыша**: Если вам повезло, вы можете вывести скин в Steam и использовать его в игре.
Чем отличается “Кейсы КС” от “Кейсы КС ГО”?
– **Кейсы КС**: Общий термин, который может относиться к кейсам в любой версии Counter-Strike, включая CS2.
– **Кейсы КС ГО**: Конкретно кейсы из CS:GO, которые были популярны до выхода CS2. Теперь многие платформы, адаптируют их под CS2, но скины из CS:GO все еще совместимы с новой игрой.
Если ты хочешь попробовать, начни с малого — внеси небольшую сумму и протестируй, как все работает. Например, выбери дешевый кейс и посмотри, что выпадет. Это поможет понять механику без больших рисков.
https://wiki.roboco.co/index.php/Cs2case_18S
Cazrfbu
| #
Мы изготавливаем дипломы психологов, юристов, экономистов и прочих профессий по приятным ценам. Купить диплом о неполном образовании — kyc-diplom.com/diplom-o-nepolnom-obrazovanii.html
Kxyufaita
| #
darknet links dark web link
w69 android app
| #
ดาวน์โหลดแอป w69 android app ง่ายๆ เพียงไม่กี่คลิก สนุกกับการเดิมพันกีฬา คาสิโน และสล็อตในที่เดียว
w69oi
| #
ดาวน์โหลดแอป w69oi
ง่ายๆ เพียงไม่กี่คลิก สนุกกับการเดิมพันกีฬา คาสิโน และสล็อตในที่เดียว
w69 เครดิตฟรี 188
| #
ติดตั้งแอป w69 เครดิตฟรี 188 แล้วรับการแจ้งเตือนแมตช์สำคัญ โปรโมชั่นพิเศษ และข่าวสารก่อนใคร
w69 asia
| #
ดาวน์โหลดแอป w69 asia แล้วเข้าถึงการเดิมพันแบบ
VIP ด้วยโปรโมชั่นและสิทธิพิเศษเฉพาะสมาชิกแอป
w69 casino login
| #
อัปเกรดแอป w69 casino login วันนี้ เพื่อการเล่นเกมที่ราบรื่นกว่าเดิม พร้อมฟังก์ชันใหม่ที่ช่วยให้คุณสนุกยิ่งขึ้น
Sheilaadurl
| #
РедМетСплав предлагает широкий ассортимент качественных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от мелких партий до крупных поставок, мы обеспечиваем своевременную реализацию вашего заказа.
Каждая единица продукции подтверждена соответствующими документами, подтверждающими их качество. Опытная поддержка – то, чем мы гордимся – мы на связи, чтобы ответить на ваши вопросы а также предоставлять решения под специфику вашего бизнеса.
Доверьте вашу потребность в редких металлах профессионалам РедМетСплав и убедитесь в множестве наших преимуществ
поставляемая продукция:
Проволока титановая Р’Рў4 Полоса титановая Р’Рў4 — это высококачественный строительный материал, обладающий отличными механическими свойствами Рё РєРѕСЂСЂРѕР·РёР№РЅРѕР№ стойкостью. Рдеально РїРѕРґС…РѕРґРёС‚ для различных отраслей, включая авиационную Рё судостроительную. Применение титана обеспечивает легкость Рё долговечность изделий. Полоса имеет стандартные размеры Рё может быть легко обработана РїРѕРґ заказ. Если РІС‹ ищете надежный материал для своего проекта, РЅРµ упустите шанс купить Полоса титановая Р’Рў4. Получите преимущества РѕС‚ использования титана Рё обеспечьте высокое качество ваших изделий.
Ronaldbut
| #
https://mycofarm.ru/
เกม สล็อต w69
| #
ดาวน์โหลดแอป เกม สล็อต w69 – https://wfi39.com ได้ง่ายๆ เพียงไม่กี่ขั้นตอน พร้อมรับโบนัสต้อนรับ 100$ สนุกกับการเดิมพันที่ดีที่สุดบนมือถือ
w69เข้าสู่ระบบ
| #
w69เข้าสู่ระบบ – https://ekn83.com ปรับปรุงฟังก์ชันแอปใหม่ ใช้งานง่ายกว่าเดิม สนุกกับคาสิโนสดและเกมสล็อตได้ทุกที่ทุกเวลา
pravo-migranta
| #
Аккредитованное агентство http://pravo-migranta.ru по аутстаффингу мигрантов и миграционному аутсорсингу. Оформление иностранных сотрудников без рисков. Бесплатная консультация и подбор решений под ваш бизнес.
Donaldlaf
| #
darknet markets onion address darkmarkets
w69 vvip
| #
แอป w69 vvip อัปเดตใหม่
โหลดเร็ว ใช้งานง่าย และรองรับภาษาไทยเต็มรูปแบบ
1win_vfMr
| #
1 вин https://1win6002.ru .
Sheilaadurl
| #
РедМетСплав предлагает обширный выбор качественных изделий из нестандартных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до масштабных поставок, мы обеспечиваем оперативное исполнение вашего заказа.
Каждая единица изделия подтверждена требуемыми документами, подтверждающими их соответствие стандартам. Опытная поддержка – наша визитная карточка – мы на связи, чтобы ответить на ваши вопросы и предоставлять решения под особенности вашего бизнеса.
Доверьте вашу потребность в редких металлах профессионалам РедМетСплав и убедитесь в широком спектре предлагаемых возможностей
поставляемая продукция:
Пруток магниевый ZK61 – CSA HG.9 Проволока магниевая ZK61 – CSA HG.9 представляет СЃРѕР±РѕР№ высококачественный РїСЂРѕРґСѓРєС‚, созданный для применения РІ различных отраслях, включая авиационную Рё автомобильную. Благодаря СЃРІРѕРёРј уникальным свойствам, таким как высокая прочность Рё легкость, эта проволока идеально РїРѕРґС…РѕРґРёС‚ для сварки Рё создания комплектующих. Если РІС‹ ищете надежный материал, чтобы улучшить СЃРІРѕРё проекты, РїРѕРєСѓРїРєР° Проволока магниевая ZK61 – CSA HG.9 станет отличным решением. РќРµ упустите возможность обеспечить СЃРІРѕСЋ продукцию великолепными характеристиками Рё долгим СЃСЂРѕРєРѕРј службы!
Toliknaf
| #
darknet market list dark web link
Pingrutty
| #
dark market dark web marketplaces
mostbet_koOt
| #
мастбет https://mostbet6007.ru/ .
Volodyanaf
| #
dark market onion best darknet markets
ktobet
| #
Cadastre-se no ktobet – https://ktobet-88.com e Receba 100$ de Bônus ao Criar Sua Conta!
1win_wiMl
| #
1win партнерская программа вход 1win партнерская программа вход .
Monitoring transporta_ibOi
| #
глонасс мониторинг транспорта личный кабинет глонасс мониторинг транспорта личный кабинет .
Kxyufaita
| #
best darknet markets darknet links
Rabyitabe
| #
dark market 2025 dark websites
bankrotstvo v moskve_btKi
| #
банкротство отзывы
От чего бывает потливость
| #
Very shortly this web site will be famous amid
all blogging and site-building people, due to it’s nice content
Lazrpgl
| #
Где заказать диплом специалиста?
Купить диплом института по доступной цене вы можете, обращаясь к надежной специализированной компании.: okdiplom.com
WilliamDix
| #
Мы изготавливаем дипломы психологов, юристов, экономистов и других профессий по выгодным ценам. Дипломы изготавливаются на подлинных бланках Приобрести диплом об образовании diplom4you.com
DonDonfaita
| #
dark web market urls darknet market links
fcb8
| #
Đăng Ký fcb8 Trên Di Động
– Đơn Giản, Nhanh Chóng
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Закупка у нас гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Обладая обширной линейкой редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается необходимыми документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым текущие трудности превращаются в завтрашние возможности.
Наша продукция:
Медно-никелевая поверхность для распыления
Kxyufaita
| #
dark web drug marketplace dark web market
Diplomi_ixka
| #
диплом о медицинском образовании купить диплом о медицинском образовании купить .
Donaldlaf
| #
darknet drug store darknet links
Pingrutty
| #
darknet markets url dark web link
zaimpodzalogptsekbsig
| #
деньги под птс сразу
avtolombard-pid-zalog-pts.ru/ekb.html
деньги под залог авто
Stephenadvic
| #
перенаправляется сюда https://xanax-futbolka.ru
Toliknaf
| #
darknet sites tor drug market
Sazrfnz
| #
Купить диплом о высшем образовании !
Приобретение диплома любого ВУЗа России у нас является надежным делом, ведь документ заносится в реестр. При этом печать осуществляется на фирменных бланках ГОЗНАКа. Купить диплом любого университета arenadiplom24.online/akademicheskie-spravki
TerryLix
| #
Все про стройку и ремонт. Актуальные интересные статьи о стройке и ремонте. Каждый найдет полезные материалы о дизайне, электрике, отоплении, кровле, сантехнике и другая полезная информация https://otoplenie-expert.com/
Ronaldbut
| #
МикоФарм Фунги
Sazrfjc
| #
Будучи студентом, я наслаждался учебой до тех пор, пока не пришло время писать диплом. Но паниковать не стоило, ведь существуют компании, которые помогают с написанием и защитой диплома на отличные оценки!
Изначально я искал информацию по теме: купить диплом о среднем образовании в краснодаре, купить диплом пту в спб, купить диплом о высшем образовании в брянске, диплом техникума купить дешево, цена аттестата, затем наткнулся на diplomybox.com/kupit-diplom-magistra-chelyabinsk
Sheilaadurl
| #
РедМетСплав предлагает широкий ассортимент высококачественных изделий из ценных материалов. Не важно, какие объемы вам необходимы – от мелких партий до масштабных поставок, мы гарантируем своевременную реализацию вашего заказа.
Каждая единица товара подтверждена требуемыми документами, подтверждающими их качество. Дружелюбная помощь – наша визитная карточка – мы на связи, чтобы разрешать ваши вопросы по мере того как предоставлять решения под специфику вашего бизнеса.
Доверьте потребности вашего бизнеса специалистам РедМетСплав и убедитесь в гибкости нашего предложения
Наша продукция:
РљСЂСѓРі магниевый M10601 – UNS Фольга магниевая M10601 – UNS представляет СЃРѕР±РѕР№ высококачественный материал, который широко используется РІ различных отраслях. РћРЅР° обладает отличной РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ устойчивостью Рё высокой прочностью, что делает её идеальной для применения РІ сложных условиях. Фольга магниевая M10601 – UNS легко обрабатывается Рё сваривается, что позволяет эффективно использовать её РІ производственных процессах. РќРµ упустите возможность купить Фольга магниевая M10601 – UNS, чтобы обеспечить надежность Рё долговечность ваших изделий. Ртот материал станет отличным решением для профессионалов Рё энтузиастов, стремящихся Рє оптимальному результату.
repraiser vaildberriz_pcmr
| #
репрайсер вайлдберриз репрайсер вайлдберриз .
Rabyitabe
| #
darknet markets url dark web link
Diplomi_ylKt
| #
Приобрести диплом любого университета!
Мы можем предложить документы любых учебных заведений, которые находятся на территории всей РФ.
diplomh-40.ru/kupit-diplom-s-registratsiej-v-reestre-nedorogo-3/
dadiwboure
| #
T.me/official_izzicasino станет для вас верным помощником и источником интересных материалов. Мы создали канал IZZI Casino в рекомендационных целях, чтобы поделиться с вами полезной информацией. Присоединяйтесь к нам и будьте в курсе горячих новостей, а также самых выгодных предложений. https://t.me/official_izzicasino/ – канал, посвященный букмекерской компании и онлайн-казино Иззи. Всегда своих игроков радуем. Выберите интересующий слот и увлекательной игрой наслаждайтесь. Попытайте счастья в выигрыше крупных призов!
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Партнерство с нашей компанией гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа любого необходимого объема.
Обладая обширной линейкой продукции на основе редкоземельных материалов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых разных заказчиков. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Приступая к взаимодействию с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым сегодняшние вызовы превращаются в завтрашние успехи.
Наша продукция:
Плоская мишень NiV
Kxyufaita
| #
bitcoin dark web dark websites
Jamesdusty
| #
Спасибо за букет невесты – всё было идеально!
заказать цветы томск
Cunokesgaw
| #
На сайте https://pilmi.ru вы найдете огромное количество фильмов самых разных жанров, включая комедии, мелодрамы, драмы, романтические. Для того чтобы подыскать наиболее подходящий вариант, нужно воспользоваться специальным каталогом. Вашему вниманию представлены и ожидаемые любопытные новинки этого года, которые произведут эффект. Все это можно просматривать в любое время и на различных устройствах. Прямо сейчас воспользуйтесь возможностью посмотреть азиатское кино. Используйте поиск для облегчения выбора.
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф гарантирует все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с высоким уровнем сервиса.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете надежность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – идеальный вариант для вас.
Наша продукция:
Меднаядвухраструбнаямуфта под пайку25х1.6ммМ1МГОСТ 32590-2013 Выберите медные двухраструбные муфты под пайку от производителя RedmetSplav. Прочные и герметичные соединения для систем водоснабжения, отопления и газоснабжения. Долговечные и надежные муфты для любых видов работ. Подходят для использования в домашних условиях, коммерческих и промышленных объектах.
DonDonfaita
| #
dark web market links darknet markets onion
Sazraqg
| #
Мы изготавливаем дипломы любых профессий по приятным тарифам.– vuz-diplom.ru/kupit-diplom-bistro-i-nadezhno-bez-lishnix-xlopot/
Pingrutty
| #
darknet market list bitcoin dark web
jafiyeordet
| #
Автосервис в СПб СТО Приморский предлагает лояльную стоимость на все услуги. Наша главная цель – обеспечить вам уверенность и комфорт на дороге. Используем качественные запчасти и современное оборудование. Запишитесь уже сегодня на ремонт автомобиля! https://sto812.com – тут есть возможность детальнее с условиями оплаты ознакомиться. Доверьте свою машину нашим специалистам. Мы даем гарантию на компетентный подход, надежность и быстрое решение любых задач. Поможем вам с подбором запчастей. Обращайтесь к нам и не пожалеете об этом!
Sheilaadurl
| #
РедМетСплав предлагает широкий ассортимент высококачественных изделий из ценных материалов. Не важно, какие объемы вам необходимы – от небольших закупок до масштабных поставок, мы обеспечиваем оперативное исполнение вашего заказа.
Каждая единица продукции подтверждена всеми необходимыми документами, подтверждающими их качество. Дружелюбная помощь – то, чем мы гордимся – мы на связи, чтобы улаживать ваши вопросы и адаптировать решения под специфику вашего бизнеса.
Доверьте потребности вашего бизнеса профессионалам РедМетСплав и убедитесь в гибкости нашего предложения
Наша продукция:
Проволока магниевая ZH62A – MIL M-46062 Полоса магниевая ZH62A – MIL M-46062 представляет СЃРѕР±РѕР№ высококачественный РїСЂРѕРґСѓРєС‚, предназначенный для использования РІ различных отраслях. Ртот материал обеспечивает отличные антикоррозийные свойства, Р° также обладает высокой прочностью Рё легкостью. Полоса магниевая ZH62A – MIL M-46062 идеально РїРѕРґС…РѕРґРёС‚ для применения РІ авиации, судостроении Рё РґСЂСѓРіРёС… областях, РіРґРµ важны надежность Рё эффективность. РќРµ упустите возможность купить Полоса магниевая ZH62A – MIL M-46062 РїРѕ выгодной цене Рё обеспечьте себе качество РЅР° долгие РіРѕРґС‹. Выбор этого продукта – это вклад РІ успешные проекты.
Sazrzmt
| #
Добрый день!
Приобрести диплом университета по невысокой стоимости можно, обращаясь к проверенной специализированной фирме. Заказать диплом: asxdiploman.com/kupit-diplom-vracha-28/
Donaldlaf
| #
darknet markets 2025 darknet websites
Cazrumv
| #
Приобрести диплом о высшем образовании!
Мы предлагаем документы университетов, которые находятся в любом регионе Российской Федерации. Дипломы и аттестаты печатаются на бумаге самого высшего качества: allboiler.ru/news/attestatu_1.html
Robertniday
| #
Все о ремонте и строительстве в ежедневных актуальных статьях. Полезные советы по ремонту, лайфхаки, интересные статьи о даче и дизайне https://premierdevelopment.ru/
Toliknaf
| #
darknet market list dark web marketplaces
Jameszem
| #
Актуальные мировые новости о политике, обществе, экномике, бизнесе, медицине и других отраслях. Ежеденевная аналитическая информация в нашем блоге https://tovarlive.ru/
EdgarKab
| #
investigate this site
russian brides for marriage
Rabyitabe
| #
darkmarkets best darknet markets
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для оформления товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы поставить любой запрос с высоким уровнем сервиса.
Наша команда поддержки всегда на связи, чтобы помочь вам в выборе товаров и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что качество и уровень нашего сервиса – наилучшее решение для вашего бизнеса.
поставляемая продукция:
Быстрорежущий Р±СЂСѓСЃРѕРє 8С…40 РјРј Р 6Рњ5Рљ5 РўРЈ РЁРёСЂРѕРєРёР№ выбор быстрорежущих Р±СЂСѓСЃРєРѕРІ для профессиональной обработки металла. Рзготовлены РёР· высокопрочных материалов, обеспечивают высокоточную работу СЃ минимальным РёР·РЅРѕСЃРѕРј. Гарантированное качество, оперативная доставка Рё профессиональная поддержка СЃРѕ стороны специалистов. РџРѕРґР±РѕСЂ оптимального инструмента для различных РІРёРґРѕРІ работ.
Source URL
| #
Its like you read my mind! You seem to know a lot about this,
like you wrote the book in it or something. I think that you could
do with a few pics to drive the message home a little bit, but instead of
that, this is fantastic blog. A great read. I will certainly be back.
TerryLix
| #
Все про стройку и ремонт. Актуальные интересные статьи о стройке и ремонте. Каждый найдет полезные материалы о дизайне, электрике, отоплении, кровле, сантехнике и другая полезная информация https://otoplenie-expert.com/
Pingrutty
| #
bitcoin dark web darknet markets onion address
Ronaldbut
| #
https://mycofarm.ru/
Mazryxl
| #
Где заказать диплом специалиста?
Мы оказываем услуги по производству и продаже документов об окончании любых университетов России. Документы производятся на подлинных бланках государственного образца. Рєradiotrainzfm301.getbb.ru/viewtopic.phpf=18&t=738
Mazrtnf
| #
Где купить диплом по актуальной специальности?
Готовый диплом с приложением отвечает стандартам Министерства образования и науки России, неотличим от оригинала – даже со специальным оборудованием. Не стоит откладывать свои цели на продолжительные годы, реализуйте их с нашей помощью – отправляйте заявку на диплом уже сегодня! Диплом о высшем образовании – не проблема! p99946c6.beget.tech/2025/03/28/kupit-diplom-bez-predoplaty-bezopasno-i-nadezhno.html
DonDonfaita
| #
darkmarket list dark market 2025
zaimpodptskemerovosig
| #
займ под птс
avtolombard-pid-zalog-pts.ru/kemerovo.html
займ под птс
Josephkaw
| #
события в мире единоборств
1win_mwOi
| #
win 1 win 1 .
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Закупка у нас гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа требуемого количества.
Имея широкий спектр редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных покупателей. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе сотрудничества. В нашем лице вы найдете не просто продавца, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Приступая к взаимодействию с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым сегодняшние вызовы превращаются в завтрашние возможности.
Наши товары:
Плоская мишень Sio для распыления
Sazrxig
| #
Приобретение документа о высшем образовании через проверенную и надежную компанию дарит немало преимуществ. Это решение дает возможность сберечь как длительное время, так и существенные финансовые средства. Однако, достоинств значительно больше.Мы изготавливаем дипломы любых профессий. Дипломы производятся на настоящих бланках. Доступная цена сравнительно с большими тратами на обучение и проживание в чужом городе. Покупка диплома университета является выгодным шагом.
Купить диплом о высшем образовании: diplomk-vo-vladivostoke.ru/bistraya-pokupka-diplomov-s-reestrom-bez-xlopot/
Cazreeo
| #
Привет!
Без университета сложно было продвигаться по карьерной лестнице. На текущий момент этот важный документ не дает совершенно никаких гарантий, что получится найти привлекательную работу. Более важны навыки специалиста, а также его опыт. Именно из-за этого решение о заказе диплома стоит считать целесообразным. Заказать диплом об образовании forum.sevsocium.ru/posting.php?mode=post&f=138&sid=94de3dcad98f4347d0084e235259a859
XunovGroup
| #
На сайте https://astro-magic.com/ воспользуйтесь искусственным интеллектом на русском языке. Этот сервис предоставляет возможность использовать все функции в режиме реального времени. При этом нет необходимости подключать VPN, искусственный интеллект работает и без него. Воспользоваться им сможет каждый желающий, абсолютно бесплатно. Chat GPT сможет создать качественный контент, произвести анализ изображений, быстро отыскать нужную информацию. Искусственный интеллект сможет и написать программный код.
Cazrehc
| #
Здравствуйте!
Мы можем предложить дипломы психологов, юристов, экономистов и прочих профессий по выгодным ценам. Стоимость может зависеть от выбранной специальности, года получения и университета: diplomanrussians.com/
Eanrpdc
| #
Для успешного продвижения вверх по карьерной лестнице требуется наличие диплома института. Приобрести диплом об образовании у проверенной компании: diplomaj-v-tule.ru/kupit-vuz-diplom-6/
Donaldlaf
| #
dark market url darkmarket 2025
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем обширный каталог продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с непревзойденным обслуживанием.
Наша команда поддержки всегда на связи, чтобы помочь вам в выборе товаров и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – идеальный вариант для вас.
поставляемая продукция:
Лента РёР· прецизионных сплавов для СѓРїСЂСѓРіРёС… элементов 0.32×35 РјРј 17ХНГТ ГОСТ 14117-85 Купите ленту РёР· прецизионных сплавов для СѓРїСЂСѓРіРёС… элементов РїРѕ выгодной цене РЅР° Редметсплав.СЂС„. Высокая прочность, устойчивость Рє РєРѕСЂСЂРѕР·РёРё Рё отличная эластичность делают этот материал идеальным для различных отраслей. Предлагаем широкий ассортимент продукции Рё РїРѕРґСЂРѕР±РЅСѓСЋ информацию для правильного выбора.
MartinSef
| #
Официальный сайт 1win http://1win.kykyryza.ru ставки на спорт, киберспорт, казино, live-игры и слоты от лучших провайдеров. Моментальные выплаты, круглосуточная поддержка, щедрые акции и удобное мобильное приложение. Делай ставки и играй в казино на 1win — быстро, безопасно и выгодно!
w69 com
| #
ดาวน์โหลดแอป w69 com – https://lvm60.com วันนี้ รับโปรโมชั่นพิเศษมากมาย ใช้งานสะดวก ปลอดภัย
และรองรับทุกอุปกรณ์
Diplomi_bfKt
| #
Купить диплом о высшем образовании!
Мы готовы предложить документы университетов, расположенных на территории всей РФ.
diplomk-vo-vladivostoke.ru/bistroe-i-nadezhnoe-vnesenie-diploma-v-reestr-2/
RogerDup
| #
Swiat emocji z 1win https://1win-pl.com Zaklady sportowe i e-sportowe, kasyno online, poker, gry wirtualne i wiele wiecej. Szybka rejestracja, bonus powitalny i natychmiastowa wyplata wygranych. 1win – wszystko, czego potrzebujesz do gry w jednym miejscu!
Lazrgsz
| #
Диплом любого ВУЗа России!
Без наличия диплома очень сложно было продвинуться вверх по карьерной лестнице. Поэтому решение о заказе диплома следует считать рациональным. Приобрести диплом о высшем образовании toolsrepair.ru/forum/viewtopic.php?f=5&t=2977031
สล็อต w69 เว็บตรง
| #
อินเทอร์เฟซใหม่ของแอป
สล็อต w69 เว็บตรง ช่วยให้การนำทางลื่นไหลขึ้น ไม่ต้องเสียเวลาค้นหาเกมโปรดของคุณ
Toliknaf
| #
darknet markets onion address dark web markets
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем внушительный выбор продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф гарантирует все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с прекрасным качеством обслуживания.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в подборе нужных изделий и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – идеальный вариант для вас.
Наши товары:
Медный стандартный двухраструбный отвод 90 градусов под пайку 14х10х0.6 мм 10.6х12.6 мм мягкая пайка М3РМ ГОСТ 32590-2013 Покупайте медные двухраструбные отводы 90 градусов под пайку на Редметсплав.рф. Надежные и долговечные отводы из высококачественной меди для систем водоснабжения, отопления и кондиционирования воздуха. Гарантированно надежные соединения, простой монтаж и оптимальное распределение потока.
Xazrhfa
| #
Заказать диплом университета!
Мы изготавливаем дипломы психологов, юристов, экономистов и любых других профессий по доступным ценам. Вы заказываете документ в надежной и проверенной компании. : myworldavto.ru/byistroe-poluchenie-diploma-ot-zakaza-do-gotovnosti
Sazrpto
| #
Мы изготавливаем дипломы любых профессий по приятным тарифам. Преимущества приобретения документов у нас
Вы приобретаете диплом через надежную и проверенную временем компанию. Такое решение сэкономит не только деньги, но и ваше драгоценное время.
На этом преимущества не заканчиваются, их гораздо больше:
• Документы изготавливаем на подлинных бланках со всеми печатями;
• Дипломы любых учебных заведений России;
• Стоимость значительно меньше чем потребовалось бы платить за обучение в ВУЗе;
• Максимально быстрая доставка в любые регионы РФ.
Купить диплом университета– http://msfo-soft.ru/msfo/forum/user/54980/ – msfo-soft.ru/msfo/forum/user/54980
Josephkaw
| #
новости шахматных турниров
MetunwAwalk
| #
На сайте https://xn—-8sbaaajsa0adcpbfha1bdjf8bh5bh7f.xn--p1ai/ ознакомьтесь со всеми вариантами антиквариата, который вы сможете продать по честной стоимости. Станислав Бабкин, являясь частным коллекционером, скупает предметы старины дорого. Оценка происходит в режиме реального времени, а средства выплачиваются на карту либо выдаются наличными. Вы сможете продать следующие вещи: серебро, картины, иконы, золотые, серебряные монеты, ордена, часы, самовары и многое другое. Вся сумма выплачивается полностью во время оформления сделки.
Sheilaadurl
| #
РедМетСплав предлагает внушительный каталог отборных изделий из редких материалов. Не важно, какие объемы вам необходимы – от небольших закупок до крупных поставок, мы гарантируем оперативное исполнение вашего заказа.
Каждая единица товара подтверждена требуемыми документами, подтверждающими их соответствие стандартам. Дружелюбная помощь – наша визитная карточка – мы на связи, чтобы улаживать ваши вопросы по мере того как предоставлять решения под особенности вашего бизнеса.
Доверьте вашу потребность в редких металлах профессионалам РедМетСплав и убедитесь в множестве наших преимуществ
Наши товары:
Пруток молибденовый РњРџР§ Пруток молибденовый РњРџР§ – это высококачественный металл, обладающий уникальными свойствами, такими как высокая температура плавления Рё стойкость Рє РєРѕСЂСЂРѕР·РёРё. Рти характеристики делают его незаменимым РІ различных отраслях, включая производство электроники Рё аэрокосмическую промышленность. Благодаря СЃРІРѕРёРј механическим свойствам, пруток идеально РїРѕРґС…РѕРґРёС‚ для изготовления деталей, подвергающихся значительным нагрузкам. Если РІС‹ хотите обеспечить надежность Рё долговечность СЃРІРѕРёС… изделий, рекомендую купить Пруток молибденовый РњРџР§, чтобы воспользоваться всеми преимуществами этого материала. Работая СЃ молибденом, РІС‹ получаете гарантированно качественный РїСЂРѕРґСѓРєС‚, который оправдает РІСЃРµ ваши ожидания.
casino Vodka официальный
| #
Добро пожаловать в Водка Казино — место, где
восторг сочетается с щедрыми бонусами и
шансами для выигрыша. У нас каждый игрок найдет свои любимые игры, будь то
начинающий или опытный игрок.
Здесь вы не только получите удовольствие, но и невероятные предложения.
Весь ассортимент игр в Водка Казино имеет высокие коэффициенты возврата, что увеличивает
шансы на выигрыш. Мы подготовили интерактивные игровые автоматы
и классические настольные игры, которые делают игровой процесс еще
более занимательным и прибыльным.
Не теряйте время! В Водка Казино вы можете быстро зарегистрироваться и легко пополнить свой счет, не тратя время на лишние шаги,
чтобы начать играть и выигрывать.
Мы всегда готовы предложить вам красивые бонусы для того, чтобы вы могли получить
больше шансов на выигрыш.
Присоединяйтесь к Водка Казино, чтобы погрузиться в мир азартных игр с высочайшими шансами на успех!
Мгновенная регистрация.
Привлекательные бонусы для новичков.
Регулярные турниры и акции для тех, кто
хочет повысить свои шансы на победу.
Круглосуточная поддержка, чтобы помочь вам в любой момент.
Играйте в любое время и в любом месте.
Погрузитесь в азартный мир Водка
Казино и проверьте свою удачу! https://vodka-casinosphere.space/
Rabyitabe
| #
darknet markets 2025 dark market
Robertniday
| #
Все о ремонте и строительстве в ежедневных актуальных статьях. Полезные советы по ремонту, лайфхаки, интересные статьи о даче и дизайне https://premierdevelopment.ru/
Pingrutty
| #
dark web market links darknet websites
Uazrckk
| #
Доброго времени суток!
Для многих людей, заказать диплом о высшем образовании – это необходимость, шанс получить отличную работу. Но для кого-то – это желание не терять время на учебу в универе. Что бы ни толкнуло вас на такой шаг, мы готовы помочь вам. Быстро, профессионально и выгодно изготовим документ нового или старого образца на подлинных бланках с реальными подписями и печатями.
Ключевая причина, почему люди покупают документы, – желание занять хорошую должность. Допустим, способности и опыт позволяют специалисту устроиться на работу, но подтверждения квалификации нет. Когда для работодателя важно наличие “корочек”, риск потерять место работы достаточно высокий.
Приобрести документ о получении высшего образования вы имеете возможность у нас в Москве. Мы предлагаем документы об окончании любых университетов России. Вы сможете получить необходимый диплом по любым специальностям, любого года выпуска, включая документы образца СССР. Гарантируем, что при проверке документа работодателями, подозрений не появится.
Обстоятельств, которые вынуждают купить диплом ВУЗа немало. Кому-то срочно требуется работа, а значит, нужно произвести впечатление на руководителя на протяжении собеседования. Другие планируют устроиться в большую компанию, для того, чтобы повысить свой статус и в будущем начать свой бизнес. Чтобы не тратить драгоценное время, а сразу начать успешную карьеру, используя врожденные таланты и приобретенные знания, можно заказать диплом через интернет. Вы станете полезным в обществе, обретете финансовую стабильность в кратчайшие сроки- диплом купить о среднем образовании
Lazropk
| #
Где приобрести диплом специалиста?
Купить диплом института по выгодной стоимости возможно, обратившись к проверенной специализированной компании.: kupite-diplom0024.ru
WilliamDix
| #
Мы изготавливаем дипломы любой профессии по приятным ценам. Дипломы производят на фирменных бланках государственного образца Приобрести диплом университета diplomd-magazinp.ru
Sazrkht
| #
Заказать диплом института по доступной цене возможно, обратившись к надежной специализированной фирме. Мы оказываем услуги по продаже документов об окончании любых университетов Российской Федерации. Заказать диплом ВУЗа– rusd-diplomj.ru/kupite-diplom-visshego-obrazovaniya-s-zaneseniem/
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф защищает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы обеспечить любой запрос с непревзойденным обслуживанием.
Наша команда поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете достоверность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – идеальный вариант для вас.
поставляемая продукция:
Титановая труба 23х1.6х3000 мм ПТ7М ГОСТ 22897-86 Откройте мир прочных и надежных титановых труб с компанией Редметсплав. Наши высококачественные титановые трубы сочетают в себе устойчивость к коррозии, износостойкость и легкий вес, делая их незаменимым материалом для применения в различных сферах промышленности. Широкий выбор различных диаметров и толщин, а также индивидуальные решения для вашего производства. Гарантированное качество и надежность поставок.
Sazrugl
| #
Купить документ университета вы имеете возможность в нашем сервисе. Мы оказываем услуги по продаже документов об окончании любых университетов России. Вы сможете получить диплом по любой специальности, включая документы образца СССР. Даем гарантию, что в случае проверки документа работодателем, подозрений не появится. diplomgorkiy.com/kupit-diplom-s-reestrom-bistro-i-udobno-3/
DonDonfaita
| #
darknet markets 2025 darknet market lists
SejehTot
| #
Welcome to the official Joy Casino blog https://officialjoycasino.net/ — a place where you can explore the fascinating world of online gambling from different perspectives: game mechanics, psychology, responsible gaming and industry analytics. If you are looking for a deep dive into gaming strategies, casino technology or responsible gaming tips, you have come to the right place.
Tuhonzoobers
| #
https://topsale.md/ – ваш надежный онлайн-гипермаркет в Молдове! Здесь вы найдете бытовую технику, электронику, товары для дома, детей и животных по выгодным ценам. Быстрая доставка по всей стране, удобные способы оплаты и акции для покупателей! Покупайте легко, экономьте время и деньги с TOPSALE.MD – ассортимент, который вам нужен, в одном месте!
Bahaxeldug
| #
На сайте https://pohudetlegko.ru/ представлена исчерпывающая, актуальная и полезная информация о том, как похудеть правильно, без вреда для организма и максимально эффективно. На сайте вы найдете вдохновляющие истории, советы звезд, которые выглядят младше своего возраста. Есть рецепты блюд, которые созданы из полезных, вкусных ингредиентов. Но при этом они помогут скинуть вес и привести себя в форму к летнему сезону. Рецепты простые и быстрые, а потому справиться с приготовлением пищи сможет каждый. Описаны и упражнения, обертывания для похудения.
1win_dvot
| #
один вин официальный сайт https://alfatraders.borda.ru/?1-0-0-00004932-000-0-0-1743258210 .
Donaldlaf
| #
darknet drug store darknet market list
Ronaldbut
| #
https://mycofarm.ru/
1win_ixOi
| #
1вин партнерка balashiha.myqip.ru/?1-12-0-00000437-000-0-0-1743258848 .
Toliknaf
| #
dark web drug marketplace dark market list
Pingrutty
| #
darknet market list dark web market urls
zaimpodptsmsksig
| #
деньги под залог автомобиля авто остается
avtolombard-pid-zalog-pts.ru
деньги под птс авто
Diplomi_poKt
| #
Заказать диплом о высшем образовании!
Мы предлагаем документы институтов, которые расположены в любом регионе Российской Федерации.
good-diplom.ru/kupit-diplom-s-zaneseniem-v-reestr-rossii-vigodno-3/
Rabyitabe
| #
dark market darkmarket
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем внушительный выбор продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы обеспечить любой запрос с прекрасным качеством обслуживания.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете достоверность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – наилучшее решение для вашего бизнеса.
Наши товары:
Латуное наружное стопорное пружинное кольцо 88С…3 РјРј ЛС59 ГОСТ 13942-86 Купить латунные наружные стопорные кольца РїРѕ ГОСТ РІ Р РѕСЃСЃРёРё. Надежная фиксация элементов конструкции. Высокое качество РїРѕ доступной цене. Рспользуются РІ строительстве, машиностроении Рё производстве. Заказывайте сейчас РЅР° сайте Редметсплав.
DonDonfaita
| #
dark web sites onion dark website
Jameszem
| #
Актуальные мировые новости о политике, обществе, экномике, бизнесе, медицине и других отраслях. Ежеденевная аналитическая информация в нашем блоге https://tovarlive.ru/
1win_suMl
| #
1 win pro 1 win pro .
Donaldlaf
| #
dark market url dark web market
Pingrutty
| #
darknet drug links darknet site
1win_voot
| #
1 вин вход 1 вин вход .
WilliamFum
| #
Ты игрок в CS2 или КС ГО? Если тебе надоели твои обыные скины и ты хочешь продать их и вывести деньги себе на карту, то для этого существуют специальные сайты, которые помогают с этим. ТУТ: вывод скинов кс ты сможешь реализовать свои скины вывести деньги на карту в течение 10 минут.
Sazrvfl
| #
Заказать диплом университета !
Приобретение диплома любого университета России в нашей компании является надежным процессом, ведь документ будет заноситься в реестр. Печать выполняется на официальных бланках ГОЗНАКа. Купить диплом об образовании arenadiplom24.online/vuzy/lipetskogo-filiala-immf
Toliknaf
| #
dark market bitcoin dark web
Sazrsjl
| #
Задался вопросом: можно ли на самом деле купить диплом государственного образца в Москве? Был приятно удивлен — это реально и легально!
Сначала искал информацию в интернете на тему: купить диплом о высшем в тюмени, купить диплом о среднем образовании в новокузнецке, купить диплом строительного техникума, купить дипломы о высшем образовании в тюмени, петербург купить диплом о высшем образовании и получил базовые знания. В итоге остановился на материале: diplomybox.com/kupit-diplom-bakalavra-v-novokuznetske
Sheilaadurl
| #
В поисках достоверного источника редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая превосходное качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для легализации товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы выполнить любой запрос с прекрасным качеством обслуживания.
Наша команда службы поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – наилучшее решение для вашего бизнеса.
Наши товары:
Латуное внутреннее стопорное пружинное кольцо 48х1.7 мм ЛЦ40С ГОСТ 13943-86 Купить латунные внутренние стопорные кольца по ГОСТ от производителя. Надежное крепление и удержание элементов механизмов. Широкий выбор размеров и конфигураций. Высокое качество и надежность. Применение в машиностроении, автомобильной и других отраслях. Обращайтесь к нашим специалистам для получения более подробной информации.
Rabyitabe
| #
dark websites best darknet markets
Ronaldbut
| #
https://mycofarm.ru/
Diplomi_ulka
| #
купить аттестат за 10 купить аттестат за 10 .
Sazrdgv
| #
Мы можем предложить дипломы психологов, юристов, экономистов и других профессий по невысоким ценам.– diplomc-v-ufe.ru/kupit-diplom-o-visshem-obrazovanii-reestr-7/
1win_fyOi
| #
1win прямой эфир 1win прямой эфир .
DonDonfaita
| #
dark web market links darknet market list
Cazrpkd
| #
Приобрести диплом любого ВУЗа!
Мы предлагаем документы институтов, которые расположены в любом регионе России. Дипломы и аттестаты делаются на бумаге самого высокого качества: s-klinika.ru/js/pgs/kupit_diplom_o_srednem_obrazovanii_30.html
Sazrxpi
| #
Добрый день!
Приобрести диплом университета по невысокой цене возможно, обратившись к проверенной специализированной компании. Купить диплом о высшем образовании: diplom-zentr.com/kupit-diplom-kemerovo-3/
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир высококлассных инноваций. Работа с нами гарантирует не только доступ к премиальным продуктам, но и возможность заказа требуемого количества.
Обладая обширной линейкой редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие стандартам качества.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Выбирая сотрудничество с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение ценными напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит достойного партнера, с которым сегодняшние вызовы превращаются в завтрашние достижения.
Наши товары:
Высокочистая металлическая мишень для распыления кремния
Pingrutty
| #
dark web drug marketplace darknet marketplace
Oscarciz
| #
Lause — fatalne miejsce do stawiania zakladow! Zgarnac nagrode jest szansa, ale uzyskac wygrana — granica nie do przekroczenia. Po uzyskaniu duzej glownej nagrody po prostu zamrozili moje konto bez zadnej informacji. Obsluga klienta milczy, a na zapytania przesylaja szablonowe odpowiedzi. Przeczytalem opinie — takich zawiedzionych sa setki! Jesli nie planujesz zmarnowac oszczednosci, omijaj szerokim lukiem na te strone!
zaimpodptsnovosibsig
| #
деньги в кредит под залог машины
avtolombard-pid-zalog-pts.ru/nsk.html
кредит под залог машины птс
Donaldlaf
| #
dark web market list darknet markets links
Toliknaf
| #
darknet sites darkmarket 2025
Sheilaadurl
| #
РедМетСплав предлагает широкий ассортимент отборных изделий из редких материалов. Не важно, какие объемы вам необходимы – от небольших закупок до обширных поставок, мы гарантируем своевременную реализацию вашего заказа.
Каждая единица товара подтверждена всеми необходимыми документами, подтверждающими их качество. Опытная поддержка – наш стандарт – мы на связи, чтобы разрешать ваши вопросы и адаптировать решения под требования вашего бизнеса.
Доверьте ваш запрос профессионалам РедМетСплав и убедитесь в гибкости нашего предложения
Наша продукция:
РљСЂСѓРі магниевый AZ91HP Фольга магниевая AZ91HP представляет СЃРѕР±РѕР№ легкий Рё прочный материал, идеально подходящий для различных промышленных Рё бытовых нужд. РћРЅР° обладает высокой РєРѕСЂСЂРѕР·РёРѕРЅРЅРѕР№ стойкостью Рё отличной пластичностью, что делает ее подходящей для изготовления сложных деталей Рё конструкций. Благодаря СЃРІРѕРёРј характеристикам, фольга широко применяется РІ авиастроении, автомобильной промышленности Рё домашних проектах. Если РІС‹ хотите обеспечить надежность Рё прочность СЃРІРѕРёС… изделий, стоит купить Фольга магниевая AZ91HP. Ртот товар станет отличным выбором для профессионалов Рё любителей.
Rabyitabe
| #
darknet market darknet markets
Pingrutty
| #
darknet drug links darknet sites
DonDonfaita
| #
darkmarket link dark web sites
Sheilaadurl
| #
В поисках надежного поставщика редкоземельных металлов и сплавов? Обратите внимание на компанию Редметсплав.рф. Мы предлагаем широкий ассортимент продукции, обеспечивая высочайшее качество каждого изделия.
Редметсплав.рф обеспечивает все стадии сделки, предоставляя полный пакет необходимых документов для законного использования товаров. Неважно, какие объемы вам необходимы – от мелких партий до крупнооптовых заказов, мы готовы обеспечить любой запрос с непревзойденным обслуживанием.
Наша команда поддержки всегда на связи, чтобы помочь вам в определении подходящих продуктов и ответить на любые вопросы, связанные с применением и характеристиками металлов. Выбирая нас, вы выбираете уверенность в каждой детали сотрудничества.
Заходите на наш сайт Редметсплав.рф и убедитесь, что наше качество и сервис – наилучшее решение для вашего бизнеса.
Наша продукция:
Однораструбный медный РѕР±РІРѕРґ РїРѕРґ пайку 22С…18С…0.9 РјРј 8С…10 РјРј твердая пайка Рњ1Рњ ГОСТ Р 52922-2008 Выбирайте высококачественные медные однораструбные РѕР±РІРѕРґС‹ РїРѕРґ пайку для надежных соединений. Рзготовлены РёР· меди высокой чистоты СЃ отличной теплопроводностью Рё устойчивостью Рє РєРѕСЂСЂРѕР·РёРё. Рдеальны для систем водоснабжения Рё отопления.
JuwadTib
| #
Спецкор Дніпро: Новини міста Дніпра і Дніпропетровської області – https://spetskor.dp.ua/ . Актуальні новини Дніпра і Дніпропетровської області. Довідкова, та корисна інформація про місто.
Ronaldbut
| #
https://mycofarm.ru/
MatthewMAind
| #
Иногда не хочется шума — просто открыть игру и разгрузить голову.
Платформа интуитивный, ничего не мешает. Выбор игр приличный, можно сменить жанр под настроение.
Депозиты заходят сразу, выплаты приходят нормально. Саппорт на связи — без лишней воды.
В целом, Игровые автоматы казино — это именно то, что надо, когда хочется просто поиграть без напряга.
Cazrwsn
| #
Здравствуйте!
Мы предлагаем дипломы любой профессии по приятным тарифам. Стоимость зависит от определенной специальности, года выпуска и ВУЗа: rdiplomm24.com/
arenda lofta v spb_bzMi
| #
лофты спб аренда для вечеринки http://www.snyat-loft-v-spb.ru .
detskaya odejda s nadpisyami_xuma
| #
одежда с надписями https://dbkids.ru/ .
Eanruve
| #
Для успешного продвижения вверх по карьерной лестнице понадобится наличие диплома о высшем образовании. Заказать диплом о высшем образовании у надежной организации: diplomg-kurerom.ru/gde-kupit-attestati-za-11-klass-nedorogo-i-bistro/
Donaldlaf
| #
tor drug market darknet markets onion address
augmented reality platform_wkSi
| #
ar gallery augmented-reality-platform.ru .
Sazraev
| #
Мы изготавливаем дипломы любой профессии по приятным тарифам. Основные преимущества заказа документов в нашем сервисе
Вы заказываете документ в надежной и проверенной компании. Такое решение позволит вам сэкономить не только много средств, но и ваше драгоценное время.
Плюсов гораздо больше:
• Документы изготавливаем на оригинальных бланках с печатями и подписями;
• Дипломы любого ВУЗа России;
• Стоимость значительно меньше той, которую понадобилось бы платить за обучение в университете;
• Доставка в любые регионы РФ.
Купить диплом о высшем образовании– http://sakhd.3nx.ru/viewtopic.php?p=3259#3259/ – sakhd.3nx.ru/viewtopic.php?p=3259#3259
Toliknaf
| #
darknet links onion dark website
Xazrgkj
| #
Заказать диплом о высшем образовании!
Мы изготавливаем дипломы любой профессии по приятным ценам. Вы заказываете диплом через надежную компанию. : matchfishing.ru/forumtest/1/member.php?u=23110
KevinKaf
| #
https://provereno-rf.ru/
Uazrkca
| #
Приветствую!
Для определенных людей, купить диплом о высшем образовании – это необходимость, уникальный шанс получить достойную работу. Но для кого-то – это желание не терять время на учебу в ВУЗе. С какой бы целью вам это не понадобилось, наша компания готова помочь вам. Быстро, профессионально и по доступной цене изготовим документ нового или старого образца на государственных бланках с реальными печатями.
Главная причина, почему люди покупают документы, – желание занять хорошую должность. Например, знания позволяют кандидату устроиться на работу, а документального подтверждения квалификации нет. В том случае если работодателю важно наличие “корочек”, риск потерять место работы очень высокий.
Заказать документ института можно в нашей компании. Мы предлагаем документы об окончании любых университетов России. Вы сможете получить необходимый диплом по любой специальности, включая документы Советского Союза. Даем 100% гарантию, что в случае проверки документа работодателем, никаких подозрений не возникнет.
Ситуаций, которые вынуждают приобрести диплом о среднем образовании очень много. Кому-то очень срочно нужна работа, и нужно произвести особое впечатление на руководителя при собеседовании. Другие желают устроиться в престижную компанию, для того, чтобы повысить собственный статус и в будущем начать свой бизнес. Чтобы не тратить драгоценное время, а сразу начать успешную карьеру, используя врожденные таланты и приобретенные навыки, можно купить диплом через интернет. Вы станете полезным в обществе, обретете денежную стабильность очень быстро и просто- купить аттестат
Pingrutty
| #
dark markets dark market link
Rabyitabe
| #
dark web market darknet drug store
ScottWeish
| #
Игровое заведение Вулкан – это захватывающий мир азарта, который поражает с первого взгляда. Благодаря прекрасному дизайну и интуитивно понятному интерфейсу, пользователи быстро оказываются в этом игровом пространстве.
Среди плюсов казино игровые автоматы следует отметить широкий ассортимент игр, включая слоты и настольные азартные игры, которые придутся по вкусу каждому игроку. Качество графики и анимации делает игровой процесс дополнительно увлекательным и завораживающим.
Однако больше всего поразил подход казино к безопасности игроков. Они используют новейшие технологии шифрования, чтобы обеспечить приватность личных и финансовых данных пользователей. Это делает казино Вулкан отличным выбором для онлайн-игры.
CumowBaify
| #
On the website https://za-forex.com/ you will find information about blockchain technology. The blog of an experienced mathematician, the one who accepted bitcoin and blockchain technology, realizing the potential of digital assets long before mainstream investors paid attention to it. He shares his knowledge to develop automated trading algorithms and risk models that can bring you profit.
KevinPiorn
| #
https://corfu-tours.ru/
zaimpodzalogptsekbsig
| #
взять деньги под птс
zaim-pod-zalog-pts1.ru/ekb.html
взять займ под залог птс
DonDonfaita
| #
darknet markets onion address dark web drug marketplace
arenda lofta v spb_ftMi
| #
аренда лофт аренда лофт .
detskaya odejda s nadpisyami_cpma
| #
одежда с надписями брендов http://dbkids.ru/ .
1win_ovpl
| #
1win казино http://1win6050.ru .
augmented reality platform_luSi
| #
augmented reality story augmented reality story .
hatori-bet
| #
hatori-bet
Sheilaadurl
| #
Сотрудничество с компанией НАПЫЛЕНИЕ РФ, специализирующейся на поставке редкоземельных материалов для различных методов напыления, открывает перед вашим предприятием двери в мир качественных инноваций. Закупка у нас гарантирует не только доступ к материалам высочайшего качества, но и возможность заказа требуемого количества.
Обладая обширной линейкой редкоземельных элементов, от мышьяка до циркония, компания НАПЫЛЕНИЕ РФ готова удовлетворить потребности самых требовательных клиентов. Каждый продукт сопровождается всеми соответствующими документами, удостоверяющими его происхождение и соответствие заданным критериям.
Кроме того, НАПЫЛЕНИЕ РФ понимает важность профессионального взаимодействия и поддержки на каждом этапе партнерских отношений. В нашем лице вы найдете не просто поставщика, но и партнера, готового предложить квалифицированную помощь и поддержку, опираться на ваши конкретные потребности и адаптировать условия поставки под особенности вашего производства.
Заключая партнерство с НАПЫЛЕНИЕ РФ, вы гарантируете себе плавное и бесперебойное снабжение необходимыми напыляемыми материалами, позволяющее вам без остановки реализовывать самые передовые и технологичные проекты. Ваш бизнес получит крепкого союзника, с которым текущие трудности превращаются в завтрашние успехи.
Наши товары:
Керамическая мишень для распыления РѕРєСЃРёРґР° олова – 4N, Плоская форма
2-bet
| #
2-bet
blogcaree
| #
Подробнее, читайте в блоге – ссылка
Donaldlaf
| #
bitcoin dark web https://github.com/aresmarketdarknetl9khn/aresmarketdarknet – dark web marketplaces
rezeki-bet
| #
rezeki-bet
1win_joEa
| #
1win казино http://www.1win6003.ru .
nissin-bet
| #
nissin-bet
mostbet_oiSr
| #
мостбет кыргызстан https://mostbet6008.ru/ .
arti-bet
| #
arti-bet
saldo-bet
| #
saldo-bet
mazda-bet
| #
http://hornoscasaemilio-valoriani.com/br/index.php?id=mazda-bet
Mazrcec
| #
Где заказать диплом специалиста?
Мы оказываем услуги по производству и продаже документов об окончании любых университетов России. Документы изготавливаются на подлинных бланках государственного образца. Рєmagnusrecruitment.com.au/employer/premialnie-diplom-24
Toliknaf
| #
onion dark website https://github.com/abacuslink4jjku/abacuslink – dark web market links
Pingrutty
| #
dark markets https://github.com/aresonioncq0a7/aresonion – dark web market list
Mazrmro
| #
Где купить диплом специалиста?
Готовый диплом с приложением 100% отвечает стандартам, никто не отличит его от оригинала. Не откладывайте свои мечты на несколько лет, реализуйте их с нашей компанией – отправьте заявку на изготовление диплома уже сегодня! Диплом о среднем образовании – не проблема! bisound.com/forum/showthread.phpp=1849429#post1849429
arenda lofta v spb_cxMi
| #
аренда лофтов спб аренда лофтов спб .
detskaya odejda s nadpisyami_iqma
| #
детская одежда с надписями http://www.dbkids.ru .
augmented reality platform_wpSi
| #
turn photo into augmented reality http://www.augmented-reality-platform.ru .
Rabyitabe
| #
darknet drugs https://github.com/darkwebsitesyhshv/darkwebsites – dark web sites